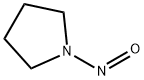
1-Nitrosopyrrolidine , Analysis standard , 930-55-2
Synonym(s):
N-Nitrosopyrrolidine;NPYR
CAS NO.:930-55-2
Empirical Formula: C4H8N2O
Molecular Weight: 100.12
MDL number: MFCD00003166
EINECS: 213-218-8
| Pack Size | Price | Stock | Quantity |
| 100MG | RMB876.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Boiling point: | 214 °C(lit.) |
| Density | 1.085 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 182 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Sparingly), Ethyl Acetate, Methanol (Slightly) |
| form | Oil |
| pka | -3.14±0.20(Predicted) |
| color | Pale Yellow to Yellow |
| CAS DataBase Reference | 930-55-2(CAS DataBase Reference) |
| IARC | 2B (Vol. 17, Sup 7) 1987 |
| EPA Substance Registry System | N-Nitrosopyrrolidine (930-55-2) |
Description and Uses
N-Nitrosopyrrolidine (NPYR) is a nitrosamine compound that is used primarily as a research chemical and is not produced commercially. There is the possibility of generating NPYR after frying in dry-cured bacon. The major amine precursors to NPYR in cooked bacon are free proline in the adipose tissue and to a lesser extent, collagenous connective tissues. When proline peptides were heated with nitrite at pH 3.4, small amounts of NPYR were formed from all tested peptides. There are several potential sources of exposure of man to this group of potent carcinogens. Certain foods were thought to be derived from the interaction of nitrite with secondary amines in the food, either spontaneously or by the agency of bacteria. Recently the possibility that nitrosamines may be formed from secondary amines and nitrite in the gastrointestinal tract has been explored. The acid conditions that prevail in the human stomach favor the nitrosation of dimethylamine. In addition, at neutral pH, many secondary amines can be nitrosated by nitrite or nitrate in the presence of intestinal bacteria, or the cecal contents of the rat, and soluble enzymes which catalyze the N-nitrosation of several secondary amines have been extracted from two microorganisms. The rate of nitrosamine formation depends greatly on the basicity of the amine; the less basic amines such as diphenylamine and pyrrolidine are nitrosated far more readily than the strongly basic dimethylamine and diethylamine.
One of the N-nitroso compounds (NOCs) implicated in human colon carcinogenesis, but the toxicological mechanisms involved have not been elucidated.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302-H351 |
| Precautionary statements | P281 |
| Hazard Codes | Xn |
| Risk Statements | 40 |
| RIDADR | 2810 |
| WGK Germany | 3 |
| RTECS | UY1575000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Hazardous Substances Data | 930-55-2(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 900 mg/kg (Druckrey) |