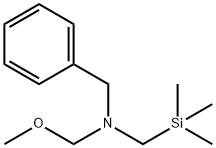
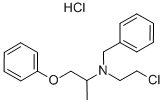
N-(Methoxymethyl)-N-(trimethylsilylmethyl)benzylamine , >90%(HPLC) , 93102-05-7
CAS NO.:93102-05-7
Empirical Formula: C13H23NOSi
Molecular Weight: 237.41
MDL number: MFCD00674005
EINECS: 630-326-4
| Pack Size | Price | Stock | Quantity |
| 1G | RMB23.20 | In Stock |
|
| 5G | RMB31.20 | In Stock |
|
| 25G | RMB108.80 | In Stock |
|
| 100g | RMB385.60 | In Stock |
|
| 500g | RMB1879.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Boiling point: | 76 °C0.3 mm Hg(lit.) |
| Density | 0.928 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 151 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in chloroform, ethyl acetate. |
| form | Liquid |
| pka | 7.29±0.50(Predicted) |
| color | Clear colorless to light yellow |
| Specific Gravity | 0.928 |
| Sensitive | Moisture & Light Sensitive |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| BRN | 4311216 |
| InChIKey | RPZAAFUKDPKTKP-UHFFFAOYSA-N |
| CAS DataBase Reference | 93102-05-7(CAS DataBase Reference) |
Description and Uses
N-Benzyl-N-(methoxymethyl)- N-trimethylsilylmethylamine (1) is a valuable reagent for in situ generation of the N-benzyl azomethine ylide (2). It is generally preferred over alternative silylmethylamine precursors6–8 because of ease of handling and use. The ylide (2) is most conveniently generated from (1) using a catalytic amount of trifluoroacetic acid as described by Achiwa.Alternative catalysts include LiF, TBAF,Me3SiOTf–CsF, or Me3SiI–CsF. Mechanistic studies provide evidence that the reactive intermediate generated from (1) with either CF3CO2H or F? is a 1,3-dipolar species. Reaction of (2) with alkenes provides an efficient convergent route to pyrrolidine derivatives. Alkynes afford 3-pyrrolines which can be converted into pyrroles.The ylide (2) reacts most readily with electron deficient alkenes and alkynes since this pairing results in a narrow dipole HOMO–dipolarophile LUMO energy gap.Examples of suitable dipolarophiles include unsaturated esters, ketones, imides,nitriles,and sulfones. Cycloaddition occurs with complete cis stereospecificity (eq 1) which is consistent with a concerted mechanism. Dipolarophiles containing an endocyclic double bond afford fused bicyclic pyrrolidines, whereas substrates with an exocyclic double bond provide access to spirocyclic systems.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39 |
| RIDADR | 1993 |
| WGK Germany | 3 |
| HazardClass | 3 |
| PackingGroup | Ⅲ |
| HS Code | 29319090 |