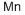Manganese , 99.95%metalsbasis , 7439-96-5
CAS NO.:7439-96-5
Empirical Formula: Mn
Molecular Weight: 54.94
MDL number: MFCD00011111
EINECS: 231-105-1
PRODUCT Properties
| Melting point: | 1244 °C (lit.) |
| Boiling point: | 1962 °C (lit.) |
| Density | 7.3 g/mL at 25 °C (lit.) |
| vapor pressure | 0-0Pa at 20℃ |
| Flash point: | 450℃ |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | Powder |
| color | Gray-brown to brown-black |
| Specific Gravity | 7.2 |
| PH | <1 (H2O, 20°C) |
| Flame Color | Yellowish green |
| Resistivity | 185 μΩ-cm, 20°C |
| Water Solubility | Soluble in diluted acids. Insoluble in water. |
| Merck | 13,5745 |
| Exposure limits | Ceiling: 5 mg(Mn)/m3 (ACGIH and OSHA)
TWA: 1 mg(Mn)/m3 (NIOSH). |
| Stability: | Stable. Incompatible with water, strong oxidizing agents, strong acids, phosphorus. |
| InChIKey | PWHULOQIROXLJO-UHFFFAOYSA-N |
| CAS DataBase Reference | 7439-96-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Manganese(7439-96-5) |
| EPA Substance Registry System | Manganese (7439-96-5) |
Description and Uses
Manganese occurs as a free element in nature and is ubiquitous in the environment. The origin and uses of this black mineral date back to the Greek golden period. Manganese was known as pyrolusite or manganese dioxide. A Swedish chemist, Carl Wilhelm Scheele, used pyrolusite to produce chlorine in the mid-eighteenth century. But not until 1774 was manganese isolated and purified as a metal by Johan Gottlieb Gahn by reducing the dioxide with carbon. Manganese is an essential element for humans and animals.
Manganese is a constituent of many alloys.It is used in the manufacture of steel. Certainmanganese compounds, such as, thefungicide, Maneb, the contrasting agent mangafodipirtrisodiumin nuclear magnetic resonancetomography for diagnostic imagingand the gasoline additive anti-knock agent,methylcyclopentadienyl manganese tricarbonyl(MMT) have been introduced into themarket in recent years.
Manganese is an essential trace elementneeded for many enzymatic reactions. Itsdeficiency is associated with growth retardation,changes in circulating HDL cholesteroland glucose levels and reproductive failure.Bone deformities have also been observedin certain animals deficient in manganese.Its deficiency, however is rarely found inhumans. Manganese is toxic at high dosesor chronic exposure. Manganese toxicity canoccur in certain occupational settings throughinhalation of its dust.
Safety
| Symbol(GHS) |  GHS02 |
| Signal word | Warning |
| Hazard statements | H228 |
| Precautionary statements | P210-P240-P241-P280-P370+P378 |
| Hazard Codes | F,Xi,T,C |
| Risk Statements | 36/38-15-11-34-23/24/25-2017/11/15 |
| Safety Statements | 45-43-26-36-36/37/39-27 |
| OEB | C |
| OEL | TWA: 1 mg/m3, STEL: 3 mg/m3 [*Note: Also see specific listings for Manganese cyclopentadienyl tricarbonyl, Methyl cyclopentadienyl manganese tricarbonyl, and Manganese tetroxide.] |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 2 |
| RTECS | OO9625000 |
| TSCA | Yes |
| HS Code | 8111 00 11 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7439-96-5(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 9000 mg/kg |
| IDLA | 500 mg Mn/m3 |