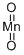Manganese dioxide , AR,85% , 1313-13-9
Synonym(s):
Birnessite manganese dioxide (δ-MnO2);Manganese dioxide;Manganese dioxide, Pyrolusite;Manganese(IV) oxide
CAS NO.:1313-13-9
Empirical Formula: MnO2
Molecular Weight: 86.94
MDL number: MFCD00003463
EINECS: 215-202-6
| Pack Size | Price | Stock | Quantity |
| 500G | RMB55.20 | In Stock |
|
| 2.5KG | RMB191.20 | In Stock |
|
| 10kg | RMB719.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 535 °C (dec.) (lit.) |
||||||||||||||
| Density | 5.02 |
||||||||||||||
| bulk density | 600-800kg/m3 |
||||||||||||||
| vapor pressure | 0-0Pa at 25℃ |
||||||||||||||
| storage temp. | Store below +30°C. |
||||||||||||||
| solubility | <0.001g/l insoluble |
||||||||||||||
| form | powder |
||||||||||||||
| color | gray |
||||||||||||||
| Specific Gravity | 5.026 |
||||||||||||||
| Water Solubility | insoluble |
||||||||||||||
| Crystal Structure | Rutile type |
||||||||||||||
| crystal system | square |
||||||||||||||
| Merck | 14,5730 |
||||||||||||||
| Space group | P42/mnm |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
||||||||||||||
| Stability: | Stable. Incompatible with strong acids, strong reducing agents, organic materials. |
||||||||||||||
| InChIKey | NUJOXMJBOLGQSY-UHFFFAOYSA-N |
||||||||||||||
| CAS DataBase Reference | 1313-13-9(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | manganese(IV) dioxide(1313-13-9) |
||||||||||||||
| EPA Substance Registry System | Manganese dioxide (1313-13-9) |
Description and Uses
Manganese dioxide is a black crystalline solid. Molecular weight= 86.94; Freezing/Melting point=(decomposes)=53℃. Hazard Identification (based on NFPA-704 M Rating System): Health 3, Flammability 0, Reactivity×(Oxidizer). Insoluble in water.
Manganese(IV) oxide is the most important ore of manganese from which the metal is mostly manufactured. The oxide occurs in nature as the mineral pyrolusite as heavy gray lumps, or black when powdered.
The mineral is used to produce manganese metal, most manganese salts, and also manganese steel and other alloys. The metallurgical applications of manganese(IV) oxide mainly involve making ferromanganese and special manganese alloys. Another important application of manganese(IV) oxide is in manufacturing dry-cell batteries and alkaline cells. The oxide also is a colorant in brick, tile, porcelain and glass; a drier for paints and varnishes; a 552 MANGANESE(IV) OXIDEpreparation for printing and dyeing textiles; a curing agent for polysulfide rubbers; an adsorbent for hydrogen sulfide and sulfur dioxide; an oxidizing agent in many organic syntheses such as quinone and hydroquinone; and a catalyst in laboratory preparation of oxygen from potassium chlorate. Manganese(IV) oxide also is used to make welding rods and fluxes, and ceramic magnets (ferrites); and is an additive to fertilizers.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H302+H332-H373 |
| Precautionary statements | P260-P264-P270-P301+P312-P304+P340+P312-P314 |
| Hazard Codes | Xn |
| Risk Statements | 20/22 |
| Safety Statements | 25 |
| RIDADR | 3137 |
| WGK Germany | 1 |
| RTECS | OP0350000 |
| TSCA | Yes |
| HS Code | 2820 10 00 |
| PackingGroup | III |
| Toxicity | LD50 orally in rats: >40 mmole/kg (Holbrook) |