A5286112
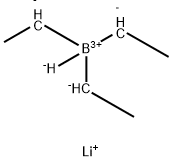
Lithium triethylborohydride , 1MinTHF , 22560-16-3
Synonym(s):
Lithium triethylborohydride solution
CAS NO.:22560-16-3
Empirical Formula: C6H13BLi
Molecular Weight: 102.92
MDL number: MFCD00011703
EINECS: 245-076-8
| Pack Size | Price | Stock | Quantity |
| 25ML | RMB125.60 | In Stock |
|
| 100ML | RMB368.80 | In Stock |
|
| 500ML | RMB1739.20 | In Stock |
|
| 800ml | RMB3359.20 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 66-67° (Binger); mp 78-83° (dec) (Brown, 1977) |
| Density | 0.892 g/mL at 25 °C |
| Flash point: | 1 °F |
| storage temp. | Flammables + water-Freezer (-20°C)e area |
| solubility | Miscible with tetrahydrofuran and benzene. |
| form | Liquid |
| color | Colorless to light gray |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,5544 |
| BRN | 4151240 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| CAS DataBase Reference | 22560-16-3(CAS DataBase Reference) |
Description and Uses
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
LiBHEt3 is a stronger reducing agent than lithium borohydride and lithium aluminium hydride.
LiTEBH can be used as a reagent:
- To reduce alkyl halides to alkanes via dehydrogenation reactions.
- For the selective reduction of epoxides to Markovnikov alcohols.
- To reduce tosylates or mesylates primary alcohols to hydrocarbons.
- For reductive cyclization reactions for the preparation of useful intermediates.
- In the synthesis of hepta(manno-3-deoxy-6-O-t-butyldimethylsilyl)-β-cyclodextrin by reduction of hepta(manno-2,3-anhydro-6-O-t-butyldimethylsilyl)-β-cyclodextrin.
- For hydrodefluorination of C-F bonds using Ni catalyst.
- To prepare alkynyl alcohols from cleavage of cyclic keto-vinyl triflates.
- In the stereoselective reduction of bicyclic imides, isoquinolines, and pyridines.
- To prepare tungsten and molybdenum hydride complexes.
Safety
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H318-H333-H225-H260-H302-H314-H335-H351 |
| Precautionary statements | P210-P231+P232-P280-P370+P378-P402+P404-P403+P235-P223-P303+P361+P353-P305+P351+P338-P405-P501a |
| Hazard Codes | F,C |
| Risk Statements | 11-14/15-19-34-40-37 |
| Safety Statements | 16-26-33-36/37/39-43-45 |
| RIDADR | UN 3399 4.3/PG 1 |
| WGK Germany | 1 |
| F | 10-23 |
| HazardClass | 4.3 |
| PackingGroup | I |
| HS Code | 29319090 |