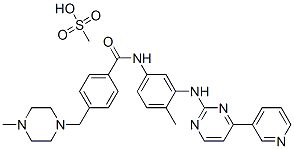
Imatinib Mesylate (STI571) , ≥99% , 220127-57-1
Synonym(s):
Imatinib mesylate;Glivec;Imatinib methanesulfonate;4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate;4-[(4-Methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide mesylate
CAS NO.:220127-57-1
Empirical Formula: C30H35N7O4S
Molecular Weight: 589.71
MDL number: MFCD04307699
EINECS: 606-892-3
| Pack Size | Price | Stock | Quantity |
| 100MG | RMB20.00 | In Stock |
|
| 250MG | RMB23.20 | In Stock |
|
| 1G | RMB53.60 | In Stock |
|
| 5g | RMB108.00 | In Stock |
|
| 25g | RMB280.80 | In Stock |
|
| 100g | RMB1119.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 214-224°C |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | White solid |
| color | white to beige |
| Water Solubility | water: 100mg/mL |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20°C for up to 3 months. |
| InChIKey | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
| SMILES | C(NC1=CC=C(C)C(NC2=NC=CC(C3=CC=CN=C3)=N2)=C1)(=O)C1=CC=C(CN2CCN(C)CC2)C=C1.CS(O)(=O)=O |
| CAS DataBase Reference | 220127-57-1(CAS DataBase Reference) |
Description and Uses
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. This compound can be prepared by a four step sequence from a condensation of the 1-(3-pyridyl)ethanone with dimethyl formamide dimethylacetal, followed by successive cyclization with the methyl-nitrophenyl guanidine, hydrogenolysis and condensation with the benzoyl chloride of the methylpiperazine. Imatinib is the first of a new class of anticancer drugs that are specifically designed to target the molecular pathways involved (oncogenic event) in the development of disease. The Brc-Abl oncoprotein is a constitutively active tyrosine kinase that causes CML. Imatinib is a competitive inhibitor of this tyrosine kinase as well as Abl, Kit and the PDGFR kinases It binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates and consequently blocks the proliferation of the leukemic cells. Phase II studies demonstrated that in chronic phase CML, over 90% of the patients had their leukocyte counts return to normal and 56% had a major cytogenic response. No phase III data is currently available. It is clear from the evidence available that imatinib has advantages over IFN-alpha, such as reduced toxicity, more rapid hematological response, higher rate of cytogenic response and oral administration. The drug is well tolerated, producing few side effects, classified as grade 1 nausea, muscle cramps, diarrhea, edema and vomiting. Imatinib is metabolized primarily by the CYP3A4 enzyme system and drugs capable of modulating this system would be expected to modify the patient's exposure. Novartis expects to launch imatinib for the treatment of gastrointestinal stromal tumors in 2002.
Imatinib Mesylate (STI571) is an orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively.
Safety
| Symbol(GHS) |  GHS08 |
| Signal word | Warning |
| Hazard statements | H361d |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| WGK Germany | 3 |
| RTECS | CV5520550 |
| HS Code | 29350090 |
| Hazardous Substances Data | 220127-57-1(Hazardous Substances Data) |