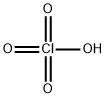
Hydrobromic acid , 48wt.%inH2O,99.99%metalsbasis , 10035-10-6
Synonym(s):
HBr;Hydrobromic acid;Hydrogen bromide in acetic acid;Hydrogen Bromide Solution, Hydrobromic Acid
CAS NO.:10035-10-6
Empirical Formula: BrH
Molecular Weight: 80.91
MDL number: MFCD00011323
EINECS: 233-113-0
PRODUCT Properties
| Melting point: | −87 °C(lit.) |
| Boiling point: | −67 °C(lit.) |
| Density | 1.49 g/mL at 25 °C (lit.) |
| vapor density | 2.8 (vs air) |
| vapor pressure | 334.7 psi ( 21 °C) |
| refractive index | n |
| Flash point: | 40°C |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| pka | -9(at 25℃) |
| form | Solution |
| color | Light yellow, brown |
| Specific Gravity | 1.49 |
| Odor | Sharp, irritating odor detectable at 2 ppm |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4778 |
| BRN | 3587158 |
| Exposure limits | Ceiling limit 3 ppm (~10 mg/m3) (ACGIH);
TLV-TWA 3 ppm (~10 mg/m3) (MSHA and
OSHA). |
| Dielectric constant | 7.0(-85℃) |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents, ammonia, ozone, fluorine, water, metals. Air and light sensitive. |
| LogP | 0.629 at 25℃ |
| CAS DataBase Reference | 10035-10-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen bromide(10035-10-6) |
| EPA Substance Registry System | Hydrobromic acid (10035-10-6) |
Description and Uses
Hydrobromic Acid is a strong acid formed by dissolving the
diatomic molecule HBr in water. “Constant-boiling”
hydrobromic acid is an aqueous solution that distills at
124.3°C and contains 47.6% HBr by weight. Hydrobromic
acid has a pKa of 9, making it a stronger acid
than hydrochloric acid, but not as strong as HI, hydroiodic
acid. Hydrobromic acid is one of the strongest
mineral acids known.
Hydrobromic acid is mainly used for the production
of inorganic bromides, especially the bromides of zinc,
calcium, and sodium. It is a useful reagent for generating
organobromine compounds. Certain ethers are
cleaved with HBr. It also catalyzes alkylation reactions
and the extraction of certain ores. Industrially significant
organic compounds prepared from hydrobromic
acid include allyl bromide, tetrabromobis(phenol),
and bromoacetic acid.
Hydrobromic acid can be prepared in the laboratory
via the reaction of Br2, SO2 and water. Another laboratory
preparation involves the production of anhydrous
HBr, which is then dissolved in water.
Hydrobromic acid has commonly been prepared
industrially by reacting bromine with either sulfur or
phosphorous and water. However, it can also be
produced electrolytically. It can also be prepared by
treating bromides with nonoxidizing acids like phosphoric
or acetic acids. Hydrobromic acid is available
commercially in various concentrations and purities.
Hydrobromic acid is used in the manufacture of bromide, as an alkylation catalyst, and in organic synthesis.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H290-H314-H335 |
| Precautionary statements | P280-P303+P361+P353-P305+P351+P338+P310 |
| Hazard Codes | C,Xi |
| Risk Statements | 35-37-34-10-36/37/38 |
| Safety Statements | 26-45-7/9-36/37/39 |
| OEL | Ceiling: 3 ppm (10 mg/m3) |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 1 |
| RTECS | MW3850000 |
| TSCA | Yes |
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28111990 |
| Hazardous Substances Data | 10035-10-6(Hazardous Substances Data) |
| Toxicity | LC50 in mice, rats: 814, 2858 ppm by inhalation, K. C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3, PB 214-270, 1972) |
| IDLA | 30 ppm |