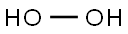Hydrogen peroxide solution , Ph.Eur.,BP,USP,30-31% , 7722-84-1
Synonym(s):
Catalase Test;Hydrogen Dioxide;Hydrogen peroxide solution;Hydrogenii peroxidum 30 per centum;Perhydrol
CAS NO.:7722-84-1
Empirical Formula: H2O2
Molecular Weight: 34.01
MDL number: MFCD00011333
EINECS: 231-765-0
PRODUCT Properties
| Melting point: | -33 °C |
| Boiling point: | 108 °C |
| Density | 1.13 g/mL at 20 °C |
| vapor density | 1.1 (vs air) |
| vapor pressure | 23.3 mm Hg ( 30 °C) |
| refractive index | 1.3350 |
| Flash point: | 107°C |
| storage temp. | 10-30°C |
| solubility | diethyl ether: soluble |
| pka | 11.5(at 25℃) |
| form | Solution |
| Specific Gravity | approximate 1.13 |
| color | ≤10(APHA) |
| PH Range | 6 - 8 at 25 °C |
| Odor | Slightly pungent, irritating odor |
| PH | 2-4 (H2O, 20°C) |
| Water Solubility | miscible |
| Merck | 14,4798 |
| BRN | 3587191 |
| Exposure limits | TLV-TWA 1 ppm (~1.5 mg/m3) (ACGIH), MSHA,andOSHA),IDLH75 ppm(NIOSH). |
| Dielectric constant | 84.2(0℃) |
| Stability: | Slightly unstable - will very slowly decompose. Decomposition is promoted by catalysts and heating, so store cool. Light sensitive, keep in the dark. May contain stabilizer. Reacts with rust, brass, zinc, nickel, finely powdered metals, copper and iron and their alloys. |
| InChIKey | MHAJPDPJQMAIIY-UHFFFAOYSA-N |
| LogP | -1.57 at 20℃ |
| CAS DataBase Reference | 7722-84-1(CAS DataBase Reference) |
| IARC | 3 (Vol. 36, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Hydrogen peroxide(7722-84-1) |
| EPA Substance Registry System | Hydrogen peroxide (7722-84-1) |
Description and Uses
Hydrogen peroxide (H2O2) is a strong oxidizing agent that is used extensively in industry and medicine. It is usually available as aqueous solutions in concentrations of 3, 30 or 90 percent by weight. The 3 percent solution is used as a topical antiseptic and cleansing agent, and as a constituent in mouthwashes, dentifrices and sanitary lotions; the 30 percent as an effective bleaching agent and for other industrial uses; and the 90 percent as a vigorous oxidizer of rocket fuels. The anhydrous form is a colorless, bittertasting liquid with an ozone-like odor. In the absence of stabilizing agents (e.g., phosphates, tin), hydrogen peroxide solutions are unstable and decompose upon standing, agitation, exposure to light, or heating. Hydrogen peroxide reacts vigorously with many oxidizing as well as reducing agents. Concentrated solutions are highly caustic to the skin. In addition to its effectiveness as a bleach, hydrogen peroxide has proved to be a useful antimicrobial agent. This latter property has been utilized in some countries as a preservative of milk and whey.
Synthesized hydrogen peroxide is approximately 60% H2O2 by weight and is distilled tohigher concentrations and diluted to lower concentrations for intended purposes. Food grade hydrogen peroxide comes in 35% and 50% concentrations. It is usedfor disinfecting purposes and also as an ingredient in cosmetics, shampoos, and medications.Reagent hydrogen peroxide for chemical and medical laboratories has a concentration of 30%.Standard grades of 35%, 50%, 60%, and 70% are used for industrial bleaching. Generalhousehold hydrogen peroxide is 3% H2O2 and 6% is used by beauticians for hair coloring.Very high grades such as 90% are used as oxidizers in rocket propulsion.
Hydrogen peroxide has a number of environmental uses. Hydrogen peroxide has a number of environmental uses. These include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation ofsubstances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.ese include water treatment, odorcontrol, oxidation of pollutants, and corrosion control. Hydrogen peroxide is used to removeiron, manganese, and hydrogen sulfide from water supplies and wastewater. The oxidation ofsubstances such as hydrogen sulfide reduces odors. Because H2O2 decomposes into oxygen andwater, it has the added advantage of lowering the biological oxygen demand of wastewater.
Hydrogen peroxide is used in chemical synthesis and can function as both an oxidizing andreducing agent. Caro’s acid (H2SO5) is made using H2O2. Peracetic acid (C2H4O3) is producedby reacting acetic acid and hydrogen peroxide and is used as a disinfectant. Solid bleachingagents such as perborates and percarbonates are made using H2O2. It is used in epoxida tionand hydroxylation reactions. Epoxidation reactions involve the breaking of double bondsin alkenes, with the carbons then bonding to the same oxygen atom to form an epoxide ring.
Safety
| Symbol(GHS) |   GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302-H315-H318-H335-H412 |
| Precautionary statements | P261-P273-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn,C,O |
| Risk Statements | 22-41-37/38-34-20/22-8-35-5 |
| Safety Statements | 26-39-45-36/37/39-28A-17-28-1/2 |
| OEB | B |
| OEL | TWA: 1 ppm (1.4 mg/m3) |
| RIDADR | UN 2014 5.1/PG 2 |
| WGK Germany | 1 |
| RTECS | MX0899500 |
| TSCA | Yes |
| HS Code | 2847 00 00 |
| HazardClass | 5.1 |
| PackingGroup | II |
| Hazardous Substances Data | 7722-84-1(Hazardous Substances Data) |
| Toxicity | LD50 oral (rat) 75 mg/kg (70%) LD50 skin (rabbit) 700 mg/kg (90%) LD50 skin (rabbit) 9200 mg/kg (70%) LC50 inhal (rat) >2000 ppm (90%) PEL (OSHA) 1 ppm (1.4 mg/m3) (90%) TLV-TWA (ACGIH) 1 ppm (1.4 mg/m3) (90%) |
| IDLA | 75 ppm |