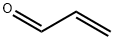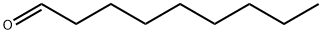Acrolein , CP , 107-02-8
Synonym(s):
2-Propenal
CAS NO.:107-02-8
Empirical Formula: C3H4O
Molecular Weight: 56.06
MDL number: MFCD00006998
EINECS: 203-453-4
PRODUCT Properties
| Melting point: | −87 °C(lit.) |
| Boiling point: | 53 °C(lit.) |
| Density | 0.839 g/mL at 25 °C(lit.) |
| vapor density | 1.94 (vs air) |
| vapor pressure | 4.05 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −2 °F |
| storage temp. | 2-8°C |
| solubility | H2O: soluble2 to 3 parts |
| form | Liquid |
| color | Colourless to Pale Yellow |
| Odor | Pungent, lacrimatory, intensely irritating odor detectable at 0.02 to 0.4 ppm |
| explosive limit | 31% |
| Odor Threshold | 0.0036ppm |
| Odor Type | fruity |
| Water Solubility | Soluble. 21.25 g/100 mL |
| Sensitive | Air & Light Sensitive |
| Merck | 14,128 |
| BRN | 741856 |
| Henry's Law Constant | (x 10-6 atmm3/mol at 25 °C):
135 (Snider and Dawson, 1985) |
| Exposure limits | NIOSH REL: TWA 0.1 ppm, STEL 0.3 ppm, IDLH 2 ppm; OSHA
PEL: TWA 0.1 ppm; ACGIH TLV: TWA 0.1 ppm, STEL 0.3 ppm. |
| Stability: | Stable, but very readily polymerizes. May have ca. 0.1% hydroquinone added as stabilizer. Flammable. Incompatible with oxidizing agents, reducing agents, oxygen, a variety of other chemicals, light. Very reactive with a wide variety of chemicals. May polymerize violently, especially on contact with strong acids or bases. |
| InChIKey | HGINCPLSRVDWNT-UHFFFAOYSA-N |
| LogP | -0.010 |
| CAS DataBase Reference | 107-02-8(CAS DataBase Reference) |
| IARC | 3 (Vol. 63, Sup 7) 1995 |
| NIST Chemistry Reference | 2-Propenal(107-02-8) |
| EPA Substance Registry System | Acrolein (107-02-8) |
Description and Uses
The first time that acrolein was produced as a commercial product was in the 1930s through the vapor-phase condensation of acetaldehyde and formaldehyde. Another method was developed in the 1940s, which involved the vapor-phase oxidation of propylene. In the 1960s, some advances were found in propylene oxidation process by the introduction of bismuth molybdate-based catalysis, and that became the primary method used for the commercial production of acrolein. Some bioproducts formed for this reaction are acrylic acid, carbon oxides, acetaldehyde, acetic acid, formaldehyde, and polyacrolein. In World War I, it was used as a chemical weapon (pulmonary irritant and lachrymatory agent). Commercial acrolein contains 95.5% or more of the compound, the main impurities being water (<3.0% by weight) and other carbonyl compounds (<1.5% by weight), mainly propanol and acetone. Hydroquinone is added as an inhibitor of polymerization (0.1–0.25% by weight).
Contact herbicide and algicide; injected in water for the control of submerged and floating weeds in irrigation ditches and canals
Safety
| Symbol(GHS) |     GHS02,GHS05,GHS06,GHS09 |
| Signal word | Danger |
| Hazard statements | H225-H300+H330-H311-H314-H410 |
| Precautionary statements | P210-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | F,T+,N,T |
| Risk Statements | 11-24/25-26-34-50-26/28-24 |
| Safety Statements | 23-26-28-36/37/39-45-61-16 |
| RIDADR | UN 1092 6.1/PG 1 |
| OEB | C |
| OEL | TWA: 0.1 ppm (0.25 mg/m3), STEL: 0.3 ppm (0.8 mg/m3) (Aldehydes) |
| WGK Germany | 3 |
| RTECS | AS1050000 |
| F | 8-9 |
| Autoignition Temperature | 234 °C |
| TSCA | TSCA listed |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 29121900 |
| Hazardous Substances Data | 107-02-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 0.046 g/kg (Smyth) |
| IDLA | 2 ppm |
| Excepted Quantities | Forbidden |