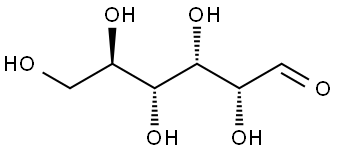
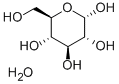
D-(+)-Glucose , standardforGC,≥99.5%(GC) , 50-99-7
Synonym(s):
D -(+)-Glucose;Dextrose;Dextrosum (Glucosum);D-Glucopyranose;D-Glucose, Corn sugar
CAS NO.:50-99-7
Empirical Formula: C6H12O6
Molecular Weight: 180.16
MDL number: MFCD00063684
EINECS: 200-075-1
| Pack Size | Price | Stock | Quantity |
| 5G | RMB63.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 150-152 °C(lit.) |
| alpha | 52.75 º (c=10, H2O, NH4OH 25 ºC) |
| Boiling point: | 232.96°C (rough estimate) |
| Density | 1.5440 |
| bulk density | 630kg/m3 |
| refractive index | 53 ° (C=10, H2O) |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | pKa 12.43(H2O,t = 18,)(Approximate) |
| form | Crystalline Powder |
| color | White |
| PH | 5.0-7.0 (25℃, 1M in H2O) |
| Odor | Odorless |
| PH Range | 5.9 |
| optical activity | [α]25/D +52.5 to +53.0°(lit.) |
| biological source | wheat |
| Water Solubility | Soluble |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,4459 |
| BRN | 1281608 |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents. Combustible. |
| InChIKey | WQZGKKKJIJFFOK-DVKNGEFBSA-N |
| LogP | -2.490 (est) |
| CAS DataBase Reference | 50-99-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Glucose(50-99-7) |
| EPA Substance Registry System | Dextrose (50-99-7) |
Description and Uses
D(+)-Glucose is one of the most important biological compounds found in nature. It is a main product in photosynthesis and is oxidized in cellular respiration. D(+)-Glucose polymerizes to form several important classes of biomolecules including cellulose, starch, and glycogen. It also combines with other compounds to produce common sugars such as sucrose and lactose. The form of D(+)-Glucose displayed above is D-D(+)-Glucose. The “D” designation indicates the configuration of the molecule. The “D” configuration specifies that the hydroxyl group on the number 5 carbon is on the right side of the molecule. The mirror image of D-D(+)-Glucose produces another form of D(+)-Glucose called L-D(+)-Glucose.D(+)-Glucose is the most common form of a large class of molecules called carbohydrates. Carbohydrates are the predominant type of organic compounds found in organisms and include sugar, starches, and fats. Carbohydrates, as the name implies, derive their name from D(+)-Glucose,C6H12O6, which was considered a hydrate of carbon with the general formula of Cn(H2O)n, where n is a positive integer. Although the idea of water bonded to carbon to form a hydrate of carbon was wrong, the term carbohydrate persisted. Carbohydrates consist of carbon, hydrogen, and oxygen atoms, with the carbon atoms generally forming long unbranched chains. Carbohydrates are also known as saccharides derived from the Latin word for sugar, saccharon.
D(+)-Glucose anhydrous for biochemistry Reag. Ph Eur. CAS 50-99-7, molar mass 180.16?g/mol.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H227 |
| Precautionary statements | P501-P270-P210-P264-P280-P370+P378-P301+P312+P330-P403+P235 |
| Hazard Codes | Xi,Xn |
| Risk Statements | 36/37/38-63-62-46-36/38-21 |
| Safety Statements | 26-36/37-24/25-53-25 |
| WGK Germany | 1 |
| RTECS | LZ6600000 |
| F | 3 |
| Autoignition Temperature | 500 °C |
| TSCA | Yes |
| HS Code | 17023051 |
| Hazardous Substances Data | 50-99-7(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 25800 mg/kg |