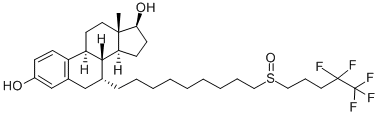
Fulvestrant , ≥99% , 129453-61-8
Synonym(s):
(7α,17β)-7-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol;Faslodex;Fulvestrant
CAS NO.:129453-61-8
Empirical Formula: C32H47F5O3S
Molecular Weight: 606.77
MDL number: MFCD00903953
EINECS: 642-998-6
| Pack Size | Price | Stock | Quantity |
| 25MG | RMB357.60 | In Stock |
|
| 250mg | RMB959.20 | In Stock |
|
| 100MG | RMB1064.00 | In Stock |
|
| 1g | RMB3039.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 104-106°C |
| Boiling point: | 674.8±55.0 °C(Predicted) |
| Density | 1.201±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >5mg/mL |
| form | powder |
| pka | 10.27±0.70(Predicted) |
| color | White |
| Stability: | Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChIKey | VWUXBMIQPBEWFH-WCCTWKNTSA-N |
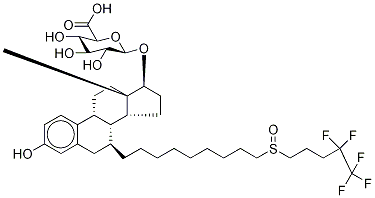
| SMILES | [C@@]12([H])[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC3C=C(O)C=CC=3[C@@]1([H])CC[C@]1(C)[C@H](CC[C@@]21[H])O |&1:0,2,32,36,38,41,r| |
| CAS DataBase Reference | 129453-61-8(CAS DataBase Reference) |
Description and Uses
Fulvestrant was launched in the US as a novel once monthly injectable steroidal estrogen antagonist for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following estrogen therapy. This 7a-alkylsulphinyl derivative of estradiol can be prepared in 10 steps from 6,7- didehydro-19-nor-testosterone by successive conjugate addition of the organocuprate derived from O-protected 9-bromononan-l-o1 followed by aromatization of the resulting enone, then activation of the protected primary alcohol, substitution with 4,4,5,5,5- pentafluoropentanthiol and oxidation to the sulfoxide. Fulvestrant is the first “pure” estrogen antagonist from a novel class known as selective estrogen receptor down regulators (SERDs). It binds to the estrogen receptor (ER), with affinity close to that of estradiol and 100 fold greater than that of tamoxifen (a partial estrogen antagonist), preventing estrogen-stimulated gene activation, thereby interfering with the estrogenrelated processes essential for cell-cycle completion. Fulvestrant also appears to downregulate the ER by 80-90% often to non detectable level both in vitro and in vivo. In comparison to tamoxifen, fulvestrant is devoid of systemic estrogenic activity, it displays no uterotrophic activity and is able to block the uterine stimulation of estradiol or tamoxifen. Furthermore, fulvestrant completely blocks the cell growth in tamoxifen-resistant breast cancer cell-lines and prevents growth of tamoxifen resistant tumor in mice. In clinical trials, it was also shown that fulvestrant is comparable to anastrozole (a third generation aromatase inhibitor) both in efficacy and tolerability in postmenopausal women with tamoxifen-resistant advanced breast cancers.
antiestrogen
Safety
| Symbol(GHS) |   GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H413 |
| Precautionary statements | P273-P501 |
| WGK Germany | 3 |
| RTECS | KG7623000 |
| HS Code | 2937230000 |
| Hazardous Substances Data | 129453-61-8(Hazardous Substances Data) |

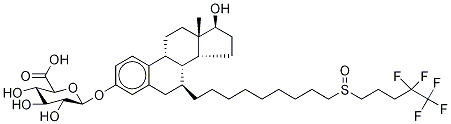
![(7a,17b)-7-7-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]-estra-1,3,5(10)-triene-3,17-diol 17-acetate](https://img.chemicalbook.com/CAS/20180808/GIF/261506-24-5.gif)