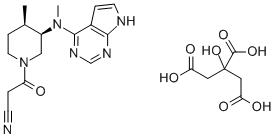
Tofacitinib (CP-690550) Citrate , ≥99% , 540737-29-9
Synonym(s):
(3R,4R)-4-Methyl-3-(methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-β-oxo-1-piperidinepropanenitrile citrate salt;3-[(3R,4R)-4-Methyl-3-[methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl]-3-oxopropanenitrile citrate salt;CP-690550-10;Tasocitinib citrate salt
CAS NO.:540737-29-9
Empirical Formula: C16H20N6O.C6H8O7
Molecular Weight: 504.497
MDL number: MFCD11616529
EINECS: 638-826-4
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB445.60 | In Stock |
|
| 1G | RMB660.00 | In Stock |
|
| 50MG | RMB1115.20 | In Stock |
|
| 5G | RMB1980.80 | In Stock |
|
| 250MG | RMB4799.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 201 °C (decomp) |
| storage temp. | room temp |
| solubility | DMSO: soluble5mg/mL (clear solution; warmed) |
| form | powder |
| color | white to beige |
| InChIKey | SYIKUFDOYJFGBQ-YLAFAASESA-N |
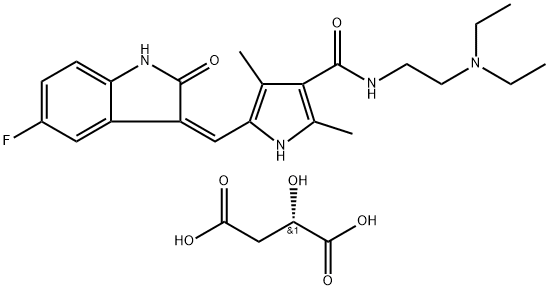
| SMILES | C1[C@@H](C)[C@@H](N(C2C3=C(NC=C3)N=CN=2)C)CN(C(CC#N)=O)C1.C(C(=O)O)C(C(=O)O)(O)CC(O)=O |&1:1,3,r| |
Description and Uses
Tofacitinib citrate, also known as CP-690550 citrate, is a king of drugs developed by the US pharmaceutical company Pfizer for treating rheumatoid arthritis, trade name Xeljanz, for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid arthritis (RA) in adult patients. This product is a Janus kinase inhibitor, administered twice daily.
November 6, 2012, the US Food and Drug Administration (FDA) and Pfizer jointly announced Tofacitinib citrate is approved for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid joints arthritis (RA) in adult patients.
Xeljanz can be used as monotherapy or in combination with methotrexate or other non-biological disease-modifying antirheumatic drugs (the DMARD) in combination. This medicine should not be in combination with biological DMARD or strong immunosuppressants (such as cyclosporine and azathioprine). Xeljanz is approved by the daily dose of 2 times, each time 5mg.
Seven clinical trials evaluated the safety and efficacy of Tofacitinib citrate in moderate to severe active RA in adult patients. In all tests, compared with patients receiving placebo, patients receiving Xeljanz treatment showed significant improvement in clinical response and physical function.
In Clinical trials, the most common adverse events were upper respiratory tract infection, headache, diarrhea, nasal congestion, sore throat, and nasopharyngitis.
Using Xeljanz was associated with an increased risk of serious infections, including opportunistic infections, tuberculosis, cancer and lymphoma. Xeljanz product label attaches boxed warning on these security risks. Xeljanz treatment is also associated with reducing blood cell counts and increasing cholesterol and liver enzyme values.
In order to study Xeljanz long-term impact on heart disease, cancer and severe infections, FDA requires for a post-marketing study, which will evaluate two doses of Xeljanz (Tofacitinib citrate) therapy, and accept a integration of another group of patients approved by the treatment as a control.
Tofacitinib citrate has been used:
as a ligand for human serum albumin in fluorescence quenching, dynamic light scattering (DLS) measurements, differential scanning calorimetry and molecular docking studies
as a medium supplement for full depth articular cartilage (FDC) explants to monitor cytokine-induced proteoglycan loss
as a Janus kinase inhibitor in MCF7 breast cancer cells
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| WGK Germany | 3 |
| HS Code | 29335990 |