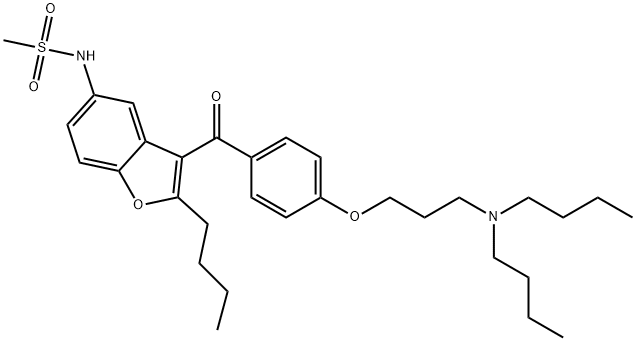
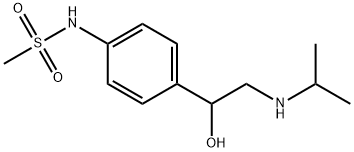
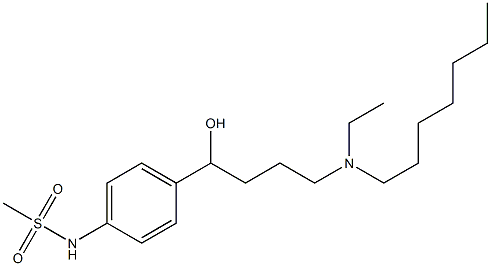
Dronedarone , ≥98% , 141626-36-0
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB259.20 | In Stock |
|
| 50MG | RMB1043.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 65.3° |
| Boiling point: | 683.9±65.0 °C(Predicted) |
| Density | 1.143±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | ≥27.84 mg/mL in DMSO; insoluble in H2O; ≥49.8 mg/mL in EtOH |
| form | solid |
| pka | 7.40±0.30(Predicted) |
| color | White to off-white |
| CAS DataBase Reference | 141626-36-0(CAS DataBase Reference) |
Description and Uses
AF is the most common form of sustained cardiac arrhythmia, with
an increasing prevalence in the aging population. AF accounts for
34.5% of arrhythmia-related hospital admissions in the United States.
The most significant consequences of AF include congestive heart failure, a 5-fold increased risk of stroke, and increased rate of mortality.
Although a 90% conversion rate from AF to normal sinus rhythm (NSR)
can be achieved with electrical cardioversion, up to 70% of these
patients require additional therapy with antiarrhythmic drugs in order
to maintain NSR.
Dronedarone, a close
analog of amiodarone, is structurally modified to provide improved
safety and pharmacokinetic profile. With the introduction of a sulfonamide group, dronedarone is less lipophilic, has lower tissue accumulation, and has a much shorter serum half-life (~24 h) compared with
amiodarone. Additionally, dronedarone lacks the iodine moieties that
are responsible for thyroid dysfunctions associated with amiodarone.
Dronedarone is specifically indicated to reduce the risk of cardiovascular hospitalization in patients with paroxysmal or persistent AF or AFL,
with a recent episode of AF/AFL and associated cardiovascular risk
factors, who are in sinus rhythm or who will be cardioverted. Similar to
amiodarone, dronedarone is a potent blocker of multiple ion currents
(including the rapidly activating delayed-rectifier potassium current,
the slowly activating delayed-rectifier potassium current, the inward rectifier potassium current, the acetylcholine-activated potassium current, peak sodium current, and L-type calcium current) and exhibits
antiadrenergic effects. Overall, dronedarone was well tolerated. The
most common side effects were gastrointestinal in nature and
included nausea, vomiting, and diarrhea.
Dronedarone-d9 is an isotopic labeled form of Dronedarone(D679445). Dronedarone is a drug used for the treatment of atrial fibrillation and atrial flutter in patients who have suffered cardiac arrhythmias.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H332-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
| Hazardous Substances Data | 141626-36-0(Hazardous Substances Data) |