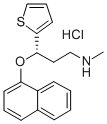
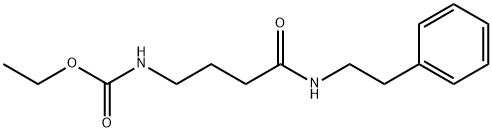
Duloxetine Hydrochloride , ≥98.0%(HPLC) , 136434-34-9
Synonym(s):
(S)-Duloxetine hydrochloride;Duloxetine hydrochloride solution;(γS)-N-Methyl-γ-(1-naphthalenyloxy)-2-thiophenepropanamine hydrochloride;(+)-(S)-N-Methyl-γ-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride;(+)-N-Methyl-3-(1-naphthalenyloxy)-3-(2-thienyl)propanamine hydrochloride
CAS NO.:136434-34-9
Empirical Formula: C18H20ClNOS
Molecular Weight: 333.88
MDL number: MFCD06407958
EINECS: 603-962-5
| Pack Size | Price | Stock | Quantity |
| 200mg | RMB103.20 | In Stock |
|
| 1G | RMB279.20 | In Stock |
|
| 5G | RMB836.00 | In Stock |
|
| 25g | RMB2851.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 118-122°C |
| Flash point: | 9℃ |
| storage temp. | room temp |
| solubility | H2O: soluble5mg/mL (clear solution, warmed) |
| pka | pKa in DMF-water (66:34): 9.6(at 25℃) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D +116 to +125°, c = 1 in methanol |
| Merck | 14,3465 |
| InChI | InChI=1/C18H19NOS.ClH/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16;/h2-10,13,17,19H,11-12H2,1H3;1H/t17-;/s3 |
| InChIKey | BFFSMCNJSOPUAY-VOPAOICTNA-N |
| SMILES | C1(=CC=CS1)[C@H](CCNC)OC1=CC=CC2=CC=CC=C12.Cl |&1:5,r| |
Description and Uses
Duloxetine is a selective serotonin (5-HT) and norepinephrine reuptake inhibitor (SSNRI) for oral administration, and it is currently approved in the US and in Europe for the treatment of major depressive disorder (MDD) and diabetic peripheral neuropathic pain (DPN). Additionally, it is indicated for the treatment of stress urinary incontinence (SUI) in Europe. However, the US approval for SUI is still pending, and Lilly recently withdrew the NDA for this indication. It is expected that additional clinical trials may be required to secure FDA approval for SUI treatment due to concern over the potential of duloxetine to prolong the QT interval in patients taking concomitant CYP2D6 or CYP1A2 inhibitors such as tricyclic antidepressants, type 1C antiarrhythmics and phenothiazines. Duloxetine is the second SSNRI to enter the market. Its predecessor, venlafaxine, has been available since 1994 for the treatment of depression. Duloxetine has a higher affinity for human norepinephrine (Ki=7.5 nM) and 5-HT transporters (Ki=0.8 nM) than venlafaxine (Ki=2480nM and 82nM, respectively). Additionally, it shows a more balanced ratio of 5-HT to norepinephrine uptake inhibition than venlafaxine. Duloxetine is a moderate inhibitor of dopamine reuptake (Ki=300nM in rat brain preparations) and shows only weak affinity for muscarinic, α1-and α2 adrenergic and histamine H1 receptors (Ki=>2.3μ). It is devoid of any activity against monoamine oxidase. In vivo, duloxetine inhibits 5-HT and norepinephrine uptake in rats, with ED50 values of 12 mg/kg and 22 mg/kg p.o., respectively, and has no effect on dopamine uptake at doses up to 30 mg/kg. The antidepressant and central paininhibitory action of SSNRIs are derived from the inhibition of both 5-HT and norepinephrine uptake, which increases the availability of neurotransmitters and ultimately increases serotonergic and noradrenergic function within the CNS. In the treatment of SUI with duloxetine, the increased neurotransmitter concentration is believed to increase the tone and contractions of the urethral sphincter at the opening of the bladder, helping to prevent accidental urine leakage. The absolute stereochemistry of duloxetine is shown to be S(+). It is prepared in four steps starting from 2-acetylthiophene. A key intermediate in the synthesis of duloxetine is (S)-3-dimethylamino-1-(2-thienyl)-1-propanol, which is obtained from the corresponding ketone, either by reduction with sodium borohydride and subsequent chiral resolution of the alcohol product with S-mandelic acid, or by enantioselective reduction with a chiral amine complex of lithium aluminum hydride. Etherification of this intermediate with 1-fluoronaphthalene, followed by N-demethylation with trichloroethylchloroformate affords duloxetine. Orally administered duloxetine is well absorbed, with Cmax achieved in 6 hours post dose. It exhibits dose-proportional pharmacokinetics at doses of 20–40 mg twice daily, with an average bioavailability of about 50% and an elimination half-life of about 12 hours. The volume of distribution is high (1943 L) and steady-state plasma levels are achieved after 3 days of dosing. Duloxetine is extensively metabolized, mainly by CYP2D6 and CYP1A2. The primary routes of elimination are in the urine (70%) and in the feces (20%). The recommended dosages of duloxetine are 20 mg to 30 mg twice daily for treating MDD, 60 mg once daily for treating DPN, and 40 mg twice daily for treating SUI. In clinical trials with adult MDD outpatients (n =1059), duloxetine at doses of 40–120 mg daily for 8 to 9 weeks demonstrated superiority over placebo as measured by improvement in the 17-item Hamilton Depression Rating Scale total score. In trials involving 791 DPN patients, 60 and 120 mg once daily dose of duloxetine statistically significantly improved the endpoint mean pain scores from baseline, and increased the proportion of patients with at least 50% reduction in pain score from baseline. All doses of duloxetine resulted in superior results than placebo. In phase III studies of adult women with predominant symptoms of SUI (n=1635), duloxetine 40 mg twice daily reduced the median incontinence episode frequency from baseline to a significantly greater extent than placebo (50.0–53.6% versus 27.5–40.0%). In addition, duloxetine recipients experienced significantly greater increases in their average voiding interval than placebo recipients (15.0–20.4 versus 1.7–8.5 minutes). The most commonly observed adverse events associated with duloxetine therapy are nausea, dry mouth, constipation, decreased appetite, fatigue, somnolence, and increased sweating. As with other 5-HT reuptake inhibitors, duloxetine is contraindicated in patients taking MAO inhibitors. Additionally, it is contraindicated in patients with uncontrolled narrowangle glaucoma.
Duloxetine Hydrochloride acts as an antidepressant and a dual serotonin and norepinephrine reuptake inhibitor (SNRI) (1,2). Exhibits linear pharmacokinetics in mice and is not associated in any drastic changes of blood pressure or heart rate (3).
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xi,Xn,T,F |
| Risk Statements | 36/37/38-22-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36/37-45-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | XN0258000 |
| HS Code | 29349990 |