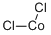Dichloromethane , ACS, ≥99.5%, containing 50-150 ppm isoprene stabilizers , 75-09-2
Synonym(s):
DCM;Dichloromethane;Methylene chloride;Dichloromethane solution;Dichloromethane ZerO2
CAS NO.:75-09-2
Empirical Formula: CH2Cl2
Molecular Weight: 84.93
MDL number: MFCD00672695
EINECS: 200-838-9
PRODUCT Properties
| Melting point: | -97 °C |
| Boiling point: | 39.8-40 °C mm Hg(lit.) |
| Density | 1.325 g/mL at 25 °C(lit.) |
| vapor density | 2.9 (vs air) |
| vapor pressure | 24.45 psi ( 55 °C) |
| refractive index | n |
| Flash point: | 39-40°C |
| storage temp. | room temp |
| solubility | Miscible in ethyl acetate, alcohol, hexanes, methanol, diethyl ether, n-octanol, acetone benzene, carbon tetrachloride, diethyl ether and chloroform. |
| form | Liquid |
| Specific Gravity | 1.329 (20/20℃) |
| color | APHA: ≤10 |
| Odor | Odor threshold 160 to 230 ppm |
| PH | 7 (20°C) |
| Odor Threshold | 160ppm |
| explosive limit | 13-22%(V) |
| Water Solubility | 20 g/L (20 ºC) |
| λmax | λ: 235 nm Amax: 1.00 λ: 240 nm Amax: 0.20 λ: 250 nm Amax: 0.05 λ: 260 nm Amax: 0.02 λ: 340-400 nm Amax: 0.01 |
| Merck | 14,6063 |
| BRN | 1730800 |
| Henry's Law Constant | 2.49 at 30 °C (headspace-GC, Sanz et al., 1997) |
| Exposure limits | TLV-TWA 50 ppm (~175 mg/m3) (ACGIH);
carcinogenicity: Suspected Human Carcinogen (ACGIH), Animal Sufficient Evidence,
Human Inadequate Evidence (IARC). |
| Dielectric constant | 9.1(20℃) |
| Stability: | Volatile |
| LogP | 1.250 |
| Surface tension | 26.5mN/m at 20°C |
| CAS DataBase Reference | 75-09-2(CAS DataBase Reference) |
| IARC | 2A (Vol. Sup 7, 71, 110) 2017 |
| NIST Chemistry Reference | Methylene chloride(75-09-2) |
| EPA Substance Registry System | Methylene chloride (75-09-2) |
| Absorption | in accordance |
Description and Uses
Dichloromethane is a colorless liquid with an ethereal, but penetrating odor. Its miscibility in alcohol and ether and slight solubility in water has made it an ideal solvent and otherwise extremely versatile chemical. It has been used industrially (solvent and paint remover), as a drug (inhalation anesthetic) and as an agricultural chemical (growth regulator and fertilizer). It is narcotic in high concentrations and carcinogenic. Inhalation exposure to this substance irritates the nose and throat and affects the central nervous system.
Methylene chloride is primarily used as a paint remover solvent. It is also used as an aerosol propellant, processing solvent in the production of steroids, antibiotics, vitamins, and tablet coatings. Additionally, it serves as a degreasing agent, particularly in electronics manufacturing. Methylene chloride is utilized as a urethane foamblowing agent as well. It finds application in metal cleaning, production of polycarbonate resins and triacetate fibers, film processing, ink formulations, and as an extraction solvent for spice oleoresins, caffeine, and hops. Due to its high volatility, it is commonly employed as a solvent in various extraction processes. Methylene chloride possesses strong solvent power for cellulose esters, fats, oils, resins, and rubber. It is more soluble in water compared to other chlorinated solvents. In the past, it was approved for use as an insecticide for commodity fumigation of strawberries, citrus fruits, and grains.
Safety
| Symbol(GHS) |   GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H315-H319-H336-H351 |
| Precautionary statements | P201-P202-P261-P302+P352-P305+P351+P338-P308+P313 |
| Hazard Codes | Xn,T,F,N,C |
| Risk Statements | 40-39/23/24/25-23/24/25-11-67-36/37/38-68/20/21/22-20/21/22-50-37-34 |
| Safety Statements | 23-24/25-36/37-45-16-7-26-61-36/37/39 |
| RIDADR | UN 1593 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | PA8050000 |
| F | 3-10 |
| Autoignition Temperature | 556 °C |
| Hazard Note | Harmful |
| TSCA | Yes |
| HS Code | 2903 12 00 |
| HazardClass | 6.1 |
| PackingGroup | III |
| Hazardous Substances Data | 75-09-2(Hazardous Substances Data) |
| Toxicity | LD50 orally in young adult rats: 1.6 ml/kg (Kimura) |
| IDLA | 2,300 ppm |