Celecoxib , ≥99% , 169590-42-5
Synonym(s):
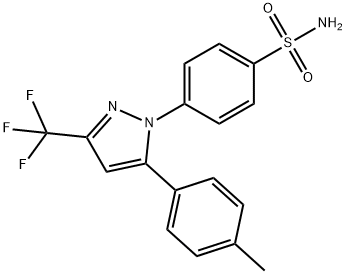
4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide;Celecoxib;YM-17
CAS NO.:169590-42-5
Empirical Formula: C17H14F3N3O2S
Molecular Weight: 381.37
MDL number: MFCD00941298
EINECS: 685-962-5
| Pack Size | Price | Stock | Quantity |
| 5G | RMB52.00 | In Stock |
|
| 25G | RMB140.80 | In Stock |
|
| 100G | RMB447.20 | In Stock |
|
| 500g | RMB1745.60 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 157-159°C |
| Boiling point: | 529.0±60.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >20mg/mL |
| pka | 9.68±0.10(Predicted) |
| form | powder |
| color | white to off-white |
| Water Solubility | 7mg/L(25 ºC) |
| Merck | 14,1956 |
| BCS Class | 2 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
| InChI | InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) |
| InChIKey | RZEKVGVHFLEQIL-UHFFFAOYSA-N |
| SMILES | C1(S(N)(=O)=O)=CC=C(N2C(C3=CC=C(C)C=C3)=CC(C(F)(F)F)=N2)C=C1 |
| CAS DataBase Reference | 169590-42-5(CAS DataBase Reference) |
| EPA Substance Registry System | Benzenesulfonamide, 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]- (169590-42-5) |
Description and Uses
Celecoxib is a nonsteroidal antiinflammatory drug (NSAID) first launched as Celebrex in the US for the treatment of symptoms in patients with rheumatoid arthritis (RA) and osteoarthritis (OA). Celecoxib belongs to a new class of 1, 5-diarylpyrazoles and can be synthesized by heat-promoted heterocyclization of a trifiuoro-l,3-dione with appropriate arylhydrazine. Celecoxib is a highly selective inhibitor of COX-2, the inducible form of cyclooxygenase expressed during inflammatory processes; it does not block the constitutive form COX-1, thus suppressing the gastric and intestinal toxicity of most non-selective NSAIDs. The potency ratio COX1/COX2 on purified human enzymes was about 400. In several in vivo models of acute and chronic inflammation, Celecoxib demonstrated potent antiinflammatory activity without affecting gastric or urinary prostaglandin PGE2. In several clinical studies performed with patients suffering from osteoarthritis or rheumatoid arthritis, Celecoxib was shown to be well tolerated and to relieve pain and inflammation more efficiently compared with other standard NSAIDs; the gastrointestinal safety profile was significantly better than that of other NSAIDs. Interestingly, Celecoxib was approved for another indication in patients with familial adenomatous polyposis (FAP). A six-month clinical trial demonstrated a 28% reduction in the number of colorectal polyps with Celecoxib, compared to a 5% reduction with placebo.
For relief and management of osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JRA), ankylosing spondylitis, acute pain, primary dysmenorrhea and oral adjunct to usual care for patients with familial adenomatous polyposis
Safety
| Symbol(GHS) |   GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H360D-H373-H410 |
| Precautionary statements | P202-P260-P273-P280-P308+P313-P391 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-52-61-60 |
| Safety Statements | 22-24/25-28-37/39 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | DB2944937 |
| HazardClass | IRRITANT |
| HS Code | 29350090 |
| Hazardous Substances Data | 169590-42-5(Hazardous Substances Data) |