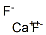Calcium fluoride , 98% , 7789-75-5
Synonym(s):
Calcium fluoride;Fluorite;Fluorspar
CAS NO.:7789-75-5
Empirical Formula: CaF2
Molecular Weight: 78.07
MDL number: MFCD00010907
EINECS: 232-188-7
| Pack Size | Price | Stock | Quantity |
| 500G | RMB68.00 | In Stock |
|
| 2.5kg | RMB287.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 1402 °C |
||||||||||||||
| Boiling point: | 2500 °C (lit.) |
||||||||||||||
| Density | 3.18 g/mL at 25 °C (lit.) |
||||||||||||||
| refractive index | 1.434 |
||||||||||||||
| Flash point: | 2500°C |
||||||||||||||
| storage temp. | -20°C |
||||||||||||||
| solubility | Slightly soluble in acid; insoluble in acetone. |
||||||||||||||
| form | rod |
||||||||||||||
| Specific Gravity | 3.18 |
||||||||||||||
| color | white |
||||||||||||||
| Odor | Odorless |
||||||||||||||
| PH | 4 (20°C, 100g/L in H2O, slurry) |
||||||||||||||
| biological source | rabbit |
||||||||||||||
| Water Solubility | 0.015 g. per 100g H20 at room temperature.(INSOLUBLE) |
||||||||||||||
| Sensitive | Hygroscopic |
||||||||||||||
| Crystal Structure | CaF2 type |
||||||||||||||
| crystal system | Cube |
||||||||||||||
| Merck | 14,1667 |
||||||||||||||
| Solubility Product Constant (Ksp) | pKsp: 8.28 |
||||||||||||||
| Space group | Fm3m |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
||||||||||||||
| Dielectric constant | 2.5(Ambient) |
||||||||||||||
| Stability: | Stable. Calcium fluoride is inert to organic chemicals and many acids, including HF. It will slowly dissolve in nitric acid. |
||||||||||||||
| Cosmetics Ingredients Functions | ORAL CARE ANTIPLAQUE |
||||||||||||||
| InChIKey | WUKWITHWXAAZEY-UHFFFAOYSA-L |
||||||||||||||
| CAS DataBase Reference | 7789-75-5(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Calcium fluoride(7789-75-5) |
||||||||||||||
| EPA Substance Registry System | Calcium difluoride (7789-75-5) |
Description and Uses
Calcium fluoride is colorless crystalline orwhite, powdery substance. Molecular weight=78.08;Specific gravity (H2O:1)=3.18 at 20℃; Boilingpoint=2495℃; Freezing/Melting point=1423℃. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 0, Reactivity 0. Practically insolublein water.
Naturally occurring CaF2 is the principal source of
hydrogenfluoride, a commodity chemicalusedto produce
a wide range of materials. Fluoride is liberated from the
mineral by the action of concentrated sulfuric acid:
CaF2 (solid)+H2SO4 (liq)→CaSO4 (solid)+2HF (gas)
CaF2 is used as an optical component because of its
chemical stability under adverse conditions.Calcium fluoride is commonly used as a window
material for both infrared and ultraviolet wavelengths,
since it is transparent in these regions (about 0.15 to
9 mm) and exhibits an extremely low-refractive index.
Calcium Fluoride is a water insoluble calcium source
for use in oxygen-sensitive applications, such as metal
production. In extremely low concentrations (ppm),
fluoride compounds are used in health applications.
Fluoride compounds also have significant uses in
synthetic organic chemistry. They are also commonly
used to alloy metal and for optical deposition.
It is also used as a flux for melting and liquid processing
of iron, steel and their composites. Its action is based
on its similar melting point to iron, on its ability to
dissolve oxides and on its ability to wet oxides and
metals.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H303-H315-H319-H335 |
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a |
| Hazard Codes | Xi,Xn |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39-36 |
| RIDADR | 3288 |
| WGK Germany | 1 |
| RTECS | EW1760000 |
| Hazard Note | Irritant |
| TSCA | Yes |
| HS Code | 28261900 |
| Hazardous Substances Data | 7789-75-5(Hazardous Substances Data) |
| Toxicity | LD50 i.p. in mice: 2638.27 mg/kg (Stratmann) |