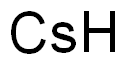Cesium , 99.8% , 7440-46-2
CAS NO.:7440-46-2
Empirical Formula: Cs
Molecular Weight: 132.91
MDL number: MFCD00134037
EINECS: 231-155-4
PRODUCT Properties
| Melting point: | 28.5 °C (lit.) |
| Boiling point: | 705 °C (lit.) |
| Density | 1.873 g/mL at 25 °C (lit.) |
| vapor pressure | 1 mm Hg ( 279 °C) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | ingot |
| color | Silver |
| Specific Gravity | 1.892 |
| PH | 0.5 (20°C in H2O) |
| Flame Color | Blue violet |
| Resistivity | 19 μΩ-cm, 0°C |
| Water Solubility | reacts with H2O to evolve H2; soluble liquid NH3 [MER06] |
| Sensitive | moisture sensitive |
| Merck | 13,2018 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Flammable solid; highly flammable in powder form. Moisture-sensitive. Incompatible with chlorine, phosphorus, water. |
| CAS DataBase Reference | 7440-46-2(CAS DataBase Reference) |
| EPA Substance Registry System | Cesium (7440-46-2) |
Description and Uses
Cesium was discovered in 1860 by Robert Bunsen and Gustav Kirchoff. It is used in the most accurate atomic clocks. Cesium melts at 28.41°C (just below body temperature) and occurs in Earth’s crust at 2.6 ppm. Cesium is the rarest of the naturally occurring alkali metals as the isotope 133Cs. Its compounds are correspondingly rare. Granites contain about 1 ppm cesium and sedimentary rocks contain approximately 4 ppm cesium. The most common commercial source of cesium is pollucite, which contains between 5 and 32% cesium oxide. Radioactive forms of cesium (134Cs and 137Cs) can also be found in the environment. They are produced during nuclear fission, and are used in cancer treatment.
Because of some of its longer-lived isotopes, cesium has become valuable for its ability toproduce a steady stream of beta particles (β) as electrons.Light is strong enough to “knock off” electrons from cesium, which makes this phenomenon useful as a coating for photoelectric cells and electric eye devices. Cesium iodide (CsI)is used in scintillation counters (Geiger counters) to measure levels of external radiation. It isalso useful as a “getter” to remove air molecules remaining in vacuum tubes.In 1960 the International Committee of Weights and Measures selected radioactive cesium-137 (with a half-life of about 33 years) as the standard for measuring time. They equated thesecond with the radiation emitted by a Cs-137 atom that is excited by a small energy source.Thus, the second is now defined as 9,192,631,770 vibrations of the radiation emitted by anatom of Cs-137. There are about 200 atomic clocks around the world that collaborate theirefforts to maintain this extremely accurate clock that never needs winding or batteries.62 | The History and Use of Our Earth’s Chemical ElementsCesium is used as a hydrogenation catalyst to enhance and assist the reaction in the conversion of liquid oils to solids forms (e.g., in the production of margarine).In a molten state, it is used as a heat-transfer fluid in plants generating electric power.Cesium is used experimentally as a plasma to produce a source of ions to power outer spacevehicles using ion engines.Cesium is used in military infrared devices and signal lamps as well as in other opticaldevices.Cesium is used as a chemical reagent and reducing agent in industry and the laboratory. Itcan also be used as an antidote for arsenic poisoning.
Safety
| Symbol(GHS) |   GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | H260-H314 |
| Precautionary statements | P223-P231+P232-P280-P305+P351+P338-P370+P378-P422 |
| Hazard Codes | Xi,C,F |
| Risk Statements | 36/38-34-14/15-11 |
| Safety Statements | 26-45-43-36/37/39-16-8 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 3 |
| RTECS | FK9225000 |
| F | 10 |
| TSCA | Yes |
| HazardClass | 4.3 |
| PackingGroup | I |
| HS Code | 28051990 |
| Hazardous Substances Data | 7440-46-2(Hazardous Substances Data) |