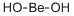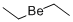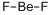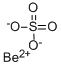Beryllium Standard , analyticalstandard,1000μg/mlin1.0mol/LHNO3 , 7440-41-7
CAS NO.:7440-41-7
Empirical Formula: BeH2
Molecular Weight: 11.03
MDL number: MFCD00134032
EINECS: 231-150-7
PRODUCT Properties
| Melting point: | 1278 °C (lit.) |
| Boiling point: | 2970 °C (lit.) |
| Density | 1.85 g/mL at 25 °C (lit.) |
| storage temp. | Store at +15°C to +25°C. |
| form | powder |
| color | Gray |
| PH | 0.5 (H2O, 20°C) |
| Flame Color | White |
| Resistivity | 4.46 μΩ-cm, 20°C |
| Water Solubility | soluble acids except HNO3; soluble alkalies [HAW93] |
| Merck | 13,1164 |
| Exposure limits | TLV-TWA 0.002 mg/m3 (ACGIH, MSHA,
and OSHA). |
| Stability: | Stable. Incompatible with acids, bases, oxidizing agents, halogen compounds, halogens, alkali metals. |
| CAS DataBase Reference | 7440-41-7(CAS DataBase Reference) |
| EPA Substance Registry System | Beryllium (7440-41-7) |
Description and Uses
Beryllium is widely distributed in the earth's crust at trace concentration, 2.8 mg/kg. The element was first discovered by Vauquelin in 1797. Wohler and Bussy in 1828 independently isolated beryllium in the metallic form from its oxide. In nature, beryllium occurs in several minerals, mostly combined with silica and alumina. The most common minerals are beryl, 3BeO•Al2O3•6SiO2; chrysoberyl, BeO•Al2O3; phenacite, 2BeO•SiO2; and bertrandite, 4BeO•2SiO2•H2O. Also, it is found in trace amounts in the ore feldspar, and in volcanic ash. It's abundance in the sea water is estimated in the range 5.6 ppt.
Beryllium oxide is a component of precious stones, emerald, aquamarine and topaz. Beryllium is utilized in nuclear reactors to moderate the velocity of slow neutrons. It is hot-pressed to appropriate shapes and sizes that yield high strength and ductility for its applications.
In the mid-twentieth century, the determination that beryllium has a number of uniqueproperties led to the production of beryllium metal by electrolysis on a commercial scale. Itproved valuable as an alloy metal to produce specialized, strong—but light—structural metalsfor use in satellites, aircraft, and spacecraft.A 2% beryllium mixture with copper produces a unique alloy of bronze that is six timesstronger than copper metal. This alloy does not give off sparks when struck with a hammer—avaluable characteristic when metals must be used in explosive gaseous environments. This alloysometimes contains small amounts of other metals such as nickel or cobalt, which makes forexcellent electrical conductivity for switching equipment, given the alloy’s simultaneous hardness and nonsparking qualities. Beryllium is also “transparent” to X-rays, which makes it idealfor windows for X-ray tubes.In 1932 James Chadwick (1891–1974) bombarded beryllium with alpha particles (heliumnuclei) that produced free neutrons. Since then, this nuclear process has made beryllium areliable neutron emitter for laboratory nuclear research. Beryllium is not only an excellentmoderator to slow down high-speed neutrons in nuclear reactors, but it also can act as areflector of neutrons as well.Beryllium is an excellent source of alpha particles, which are the nuclei of helium atoms.Alpha particles (radiation) are not very penetrating. These particles travel only a few inchesin air and can be stopped by a sheet of cardboard. Alpha particles are produced in cyclotrons(atom smashers) and are used to bombard the nuclei of other elements to study their characteristics.In the first part of the twentieth century, beryllium was used as coating inside fluorescentelectric light tubes, but proved carcinogenic (causes cancer) when broken tubes producedberyllium dust that was inhaled. Because of this potential to cause cancer, since 1949 beryllium has no longer been used as the inside coating of fluorescent tubes. Beryllium is alsoused for computer parts, electrical instrument components, and solid propellant rocket fuels.Because it is one of the few metals that is transparent to X-rays, it is used to make special glassfor X-ray equipment.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301-H315-H317-H319-H330-H335-H350i-H372-H350 |
| Precautionary statements | P301+P310a-P304+P340-P305+P351+P338-P320-P330-P405-P501a-P201-P260-P280-P284-P301+P310+P330-P304+P340+P310 |
| Hazard Codes | T+,T |
| Risk Statements | 49-25-26-36/37/38-43-48/23-20 |
| Safety Statements | 53-45 |
| RIDADR | UN 1567 6.1/PG 2 |
| WGK Germany | 1 |
| RTECS | DS1750000 |
| Autoignition Temperature | 1198 °F |
| TSCA | Yes |
| HS Code | 3822 00 00 |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-41-7(Hazardous Substances Data) |
| Toxicity | Elemental Be and its compounds are very poisonous by inhalation or intravenous route. Chronic inhalation of beryllium dusts or fumes can cause a serious lung disease, berylliosis, after a latent period ranging from several months to many years. Inhalation of airborne dusts can also cause an acute disease manifested as dyspnea, pneumonitis and tracheobronchitis with a short latency period of a few days. Skin contact with soluble salts of the metal can cause dermatitis. Beryllium also is a carcinogen. There is sufficient evidence of its inducing cancer in animals and humans. It is one of the US EPA's listed priority pollutant metals in the environment. |
| IDLA | 4 mg Be/m3 |