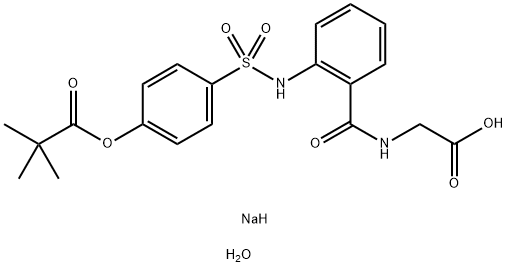
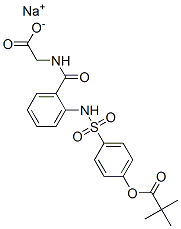
Sivelestatsodiumtetrahydrate , ≥98% , 201677-61-4
CAS NO.:201677-61-4
Empirical Formula: C20H29N2NaO11S
Molecular Weight: 528.51
MDL number: MFCD01937969
| Pack Size | Price | Stock | Quantity |
| 5mg | RMB277.60 | In Stock |
|
| 10mg | RMB503.20 | In Stock |
|
| 25mg | RMB1023.20 | In Stock |
|
| 50mg | RMB1727.20 | In Stock |
|
| 100mg | RMB2519.20 | In Stock |
|
| 250mg | RMB4159.20 | In Stock |
|
| 1g | RMB11647.20 | In Stock |
|
| 5g | RMB31999.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO (up to 20 mg/ml). |
| form | White solid. |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months |
| InChIKey | YMOIFFHCERRYCO-UHFFFAOYSA-N |
| SMILES | C(C1C=CC=CC=1NS(C1C=CC(OC(=O)C(C)(C)C)=CC=1)(=O)=O)(=O)NCC(=O)O.[NaH].O |
| CAS DataBase Reference | 201677-61-4(CAS DataBase Reference) |
Description and Uses
Sivelestat is an acyl enzyme inhibitor of neutrophil elastase, developed as an injectable formulation for the treatment of acute lung injury associated with systemic inflammatory response syndrome. A neutrophil predominant inflammation associated with excessive release of human neutrophil elastase (HNE) from azurophilic granules is capable of damaging both the lung parenchymal cells and the extracellular matrix allowing an alveolar capillary barrier disruption. Sivelestat is a sulfonanilide-containing pivaloyloxy benzene derivative prepared in a three step synthesis. This agent acts as a reversible and selective inhibitor of HNE with an lG.0 value of 0.044 PM. Sivelestat has exhibited potent protective effects against various causes of lung injuries in animal models. In an acid-induced acute lung injury model in conscious hamster, administration of sivelestat for 48 h following HCI instillation, dose-dependently reduced mortality and significantly improved the protein levels in bronchoalveolar lavage fluids and pulmonary artery pressure. In a similar study, the agent inhibited the endotoxin-induced acute lung dysfunctions (marked elevation of pulmonary vascular permeability, leukocyte migration, hemorrhage and parenchymal injury) in different animal species. Moreover, in a cardiopulmonary bypass model in dog, sivelestat ameliorated the respiratory index and interstitial-intra-alveolar edema. Sivelestat has a relatively poor bioavailability, due to an extensive first-pass metabolism and is easily hydrolyzed in vitro to an inactive metabolite. In two animal species, the half-life time was approximately 5-7 min. Clinical trials have shown that treatment with the agent improves respiratory function and facilitates early removal of patients from mechanical ventilation. However, in the last clinical study, conducted by Eli Lilly in patients with acute lung injury, no difference in mortality and safety was seen between sivelestat and placebo.
Treatment of acute lung injury; acute respiratory distress syndrome (elastase inhibitor).
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 2935909099 |