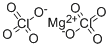Magnesium perchlorate , ACS, dry level, for water absorption , 10034-81-8
CAS NO.:10034-81-8
Empirical Formula: 2ClO4.Mg
Molecular Weight: 223.2
MDL number: MFCD00274167
EINECS: 233-108-3
PRODUCT Properties
| Melting point: | 251 °C |
| Density | 2,21 g/cm3 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Hygroscopic, Room Temperature, Under inert atmosphere |
| solubility | Ethanol (Slightly), Water (Slightly) |
| form | Solid |
| Specific Gravity | 2.21 |
| color | White |
| Water Solubility | Magnesium perchlorate is soluble in water, ethanol. |
| Sensitive | Hygroscopic |
| Merck | 14,5678 |
| Stability: | Stable, but moisture sensitive. Oxidizer - contact with combustible material may lead to fire. Incompatible with reducing agents, organic materials, trimethyl phosphate, powdered metals, strong acids, phosphorus. |
| LogP | -6.53 at 25℃ |
| CAS DataBase Reference | 10034-81-8(CAS DataBase Reference) |
| EPA Substance Registry System | Perchloric acid, magnesium salt (2:1) (10034-81-8) |
Description and Uses
Magnesium perchlorate is a powerful oxidizing
agent, with the formula Mg(ClO4)2. Magnesium perchlorate
decomposes at 250°C. The heat of formation is 568.90 kJ/mol. If water is added to magnesium perchlorate, the reaction
is highly exothermic and may cause damage to the
eyes. Thus, this salt should be added to a sufficient
amount of water in order to form a dilute solution.
Mg(ClO4)2·6H2O is composed of white, deliquescent
crystals which are soluble in water and alcohol but explosive when in contact with reducing materials.
This compound is used as a drying agent for gases.
This salt is highly exothermic when added to water
and releases steamy HCl gas. It can be dried to form
the anhydrate by heating to 250°C under vacuum. The
hexahydrate losses two molecules of water at 244°C in
air and further decomposes to the dihydrate at 336°C
and the anhydrate at 438°C.
The anhydrate has been used as a desiccant to dry gas
streams or air samples. However, it is no longer used
since perchlorates tend to form explosive compounds
with a variety of organic materials as well as other
substances.
Magnesium perchlorate is commonly utilized as a potent oxidizing agent. It is used as dehydrating agent in organic synthesis. It is practiced in many domains like pharma industry, pyrotechnics industry,in rechargeable batteries and it is a versatile tool in organic synthesis. It possesses photoelectronic and optical properties. Thus it can be used in photo electronic and optoelectronic devices. It is applied as a strong drying agent for gases.
Safety
| Symbol(GHS) |   GHS03,GHS07 |
| Signal word | Danger |
| Hazard statements | H272-H315-H319-H335 |
| Precautionary statements | P210-P302+P352-P305+P351+P338 |
| Hazard Codes | O,Xi |
| Risk Statements | 8-36/37/38-14/15 |
| Safety Statements | 17-26-27-36/37/39-43-7/8 |
| RIDADR | UN 1475 5.1/PG 2 |
| WGK Germany | 3 |
| RTECS | SC8925000 |
| F | 3-10 |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | II |
| HS Code | 28299010 |
| Hazardous Substances Data | 10034-81-8(Hazardous Substances Data) |
| Toxicity | LD50 ipr-mus: 1500 mg/kg JAFCAU 14,512,66 |