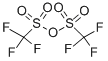
Trifluoromethanesulfonicanhydride , ≥99% , 358-23-6
Synonym(s):
Perfluoromethanesulfonic anhydride solution;Tf2O;Triflic anhydride;Triflic anhydride solution;Trifluoromethanesulfonic anhydride
CAS NO.:358-23-6
Empirical Formula: C2F6O5S2
Molecular Weight: 282.14
MDL number: MFCD00000408
EINECS: 206-616-8
| Pack Size | Price | Stock | Quantity |
| 10g | RMB295.20 | In Stock |
|
| 25g | RMB511.20 | In Stock |
|
| 250g | RMB2215.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | -80°C |
| Boiling point: | 81-83 °C (lit.) |
| Density | 1.677 g/mL at 25 °C (lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 8 mm Hg ( 20 °C) |
| refractive index | n |
| RTECS | PB2772000 |
| Flash point: | 81-83°C |
| storage temp. | Store below +30°C. |
| solubility | Miscible with dichloromethane. Immiscible with hydrocarbons. |
| form | Liquid |
| Specific Gravity | 1.677 |
| color | Clear colorless to light brown |
| Water Solubility | reacts violently with water |
| Sensitive | Moisture Sensitive |
| BRN | 1813600 |
| Stability: | Hygroscopic, Moisture Sensitive |
| InChIKey | WJKHJLXJJJATHN-UHFFFAOYSA-N |
| LogP | 0.3 at 25℃ |
| CAS DataBase Reference | 358-23-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Trifluoromethanesulfonic anhydride(358-23-6) |
| EPA Substance Registry System | Methanesulfonic acid, trifluoro-, anhydride (358-23-6) |
Description and Uses
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, has proven to be an extraordinary reagent for a broad range of transformations. As a commercially and readily available reagent, It has been widely used in synthetic chemistry due to its high electrophilicity. Given its high affinity towards O-nucleophiles, reaction with alcohols, carbonyls, sulfur phosphorus- and iodine oxides towards the formation of the corresponding triflates is strongly favored. As one of the premier leaving groups in organic chemistry, the generated triflates then open the door to various downstream transformations, including (but not limited to) substitution reactions, cross-coupling processes, redox reactions, and rearrangements[1-2].
Trifluoromethanesulfonic anhydride is used to convert phenols and imine into triflic ester and NTf group. It is a strong electrophile used for the introduction of triflyl group in chemical synthesis. It serves as a reagent in the preparation of alkyl and vinyl triflates, and for the stereoselective synthesis of mannosazide methyl uronate donors. It acts as a catalyst for glycosylation with anomeric hydroxy sugars to prepare polysaccharides.
Safety
| Symbol(GHS) |    GHS03,GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H272-H302-H314-H335 |
| Precautionary statements | P210-P220-P280-P301+P312-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | C |
| Risk Statements | 14-21/22-34-35-22-40 |
| Safety Statements | 26-36/37/39-43-45-8 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| F | 10-21 |
| Hazard Note | Corrosive |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | I |
| HS Code | 29049020 |
| Toxicity | LD50 orally in Rabbit: 1012 mg/kg |