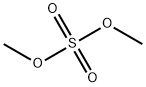
Dimethyl Sulfate , >98.0%(GC) , 77-78-1
Synonym(s):
Sulfuric acid dimethyl ester
CAS NO.:77-78-1
Empirical Formula: C2H6O4S
Molecular Weight: 126.13
MDL number: MFCD00008416
EINECS: 201-058-1
PRODUCT Properties
| Melting point: | -32 °C |
| Boiling point: | 188 °C(lit.) |
| Density | 1.333 g/mL at 25 °C(lit.) |
| vapor density | 4.3 (vs air) |
| vapor pressure | 0.7 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 182 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: 0.26 g/mL, clear, colorless |
| form | Liquid |
| color | Clear colorless |
| Odor | Almost odorless |
| explosive limit | 3.6-23.2% (v/v) |
| Water Solubility | 2.8 g/100 mL (18 ºC) |
| Merck | 13,3282 |
| BRN | 635994 |
| Exposure limits | TLV/PEL-TWA skin 0.1 ppm (0.52 mg/m3 )
(ACGIH, OSHA, NIOSH)
IDLH 10 ppm (NIOSH). |
| Dielectric constant | 55.0(20℃) |
| Stability: | Stable; combustible. Incompatible with strong oxidizing agents, strong bases including ammonia. Moisture-sensitive. |
| CAS DataBase Reference | 77-78-1(CAS DataBase Reference) |
| IARC | 2A (Vol. 4, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Sulfuric acid, dimethyl ester(77-78-1) |
| EPA Substance Registry System | Dimethyl sulfate (77-78-1) |
Description and Uses
Dimethyl sulfate is a colorless, oily liquid with a faint, onionlike
odor. It is soluble in water, ether, dioxane, acetone,
benzene, and other aromatic hydrocarbons, miscible with
ethanol, and sparingly soluble in carbon disulfide. It is stable
under normal temperatures and pressures, but hydrolyzes
rapidly in water at or above 18 ℃.
Dimethyl sulfate has been produced commercially since at
least the 1920s. One production method is continuous reaction
of dimethyl ether with sulfur trioxide. In 2009, dimethyl sulfate
was produced by 33 manufacturers worldwide, including 1 in
the United States, 14 in China, 5 in India, 5 in Europe, 6 in East
Asia, and 2 in Mexico, and was available from 44 suppliers,
including 16 US suppliers. There are no data on US imports or
exports of dimethyl sulfate. Reports filed from 1986 through
2002 under the US Environmental Protection Agency’s Toxic
Substances Control Act Inventory Update Rule indicate that US
production plus imports of dimethyl sulfate totaled 10–50
million pounds. The simplest way of synthesizing dimethyl
sulfate is by esterification of sulfuric acid with methanol as
follows:2CH3OH+ H2SO4→(CH3)2SO4 + 2H2O
Dimethyl sulfate is a strong alkylating agent and might also react with the carboxylic acid substrate, further reducing the DMS concentration in the mixture. It is used as a methylating agent in themanufacture of many organic compounds,such as, phenols and thiols. Also, it is used inthe manufacture of dyes and perfumes, andas an intermediate for quaternary ammoniumsalts. It was used in the past as a militarypoison.
Safety
| Symbol(GHS) |    GHS05,GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H301-H314-H317-H330-H341-H350 |
| Precautionary statements | P202-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T+ |
| Risk Statements | 45-25-26-34-43-68 |
| Safety Statements | 53-45-61 |
| RIDADR | UN 1595 6.1/PG 1 |
| OEB | C |
| OEL | TWA: 0.1 ppm (0.5 mg/m3) [skin] |
| WGK Germany | 2 |
| RTECS | WS8225000 |
| F | 21 |
| Autoignition Temperature | 495 °C |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 29209090 |
| Hazardous Substances Data | 77-78-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 440 mg/kg (Smyth) |
| IDLA | 7 ppm |