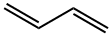1,3-Butadiene (ca. 15% in Hexane) , 106-99-0
CAS NO.:106-99-0
Empirical Formula: C4H6
Molecular Weight: 54.09
MDL number: MFCD00008659
EINECS: 203-450-8
PRODUCT Properties
| Melting point: | −109 °C(lit.) |
| Boiling point: | −4.5 °C(lit.) |
| Density | 0.62 g/mL at 20 °C(lit.) |
| vapor density | 1.9 (15 °C, vs air) |
| vapor pressure | 1863 mm Hg ( 21 °C) |
| refractive index | 1.4292 |
| Flash point: | −105 °F |
| storage temp. | 0-6°C |
| solubility | water: soluble0.5g/L at 20°C |
| form | Colorless gas |
| color | Colorless to Almost colorless |
| Odor Threshold | 0.23ppm |
| explosive limit | 12% |
| Water Solubility | 735mg/L(25 ºC) |
| FreezingPoint | -108.91℃ |
| Merck | 14,1509 |
| BRN | 605258 |
| Henry's Law Constant | (x 10-2 atm?m3/mol):
6.3 at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | TLV-TWA 10 ppm (~22 mg/m3) (ACGIH),
1000 ppm (OSHA and NIOSH); IDLH
20,000 ppm (NIOSH); A2–Suspected Human
Carcinogen (ACGIH). |
| Stability: | Stable. Extremely flammable. May form explosive mixtures with air. Incompatible with strong oxidizing agents, copper, copper alloys. May contain stabilizer. |
| InChIKey | KAKZBPTYRLMSJV-UHFFFAOYSA-N |
| LogP | 1.99 at 20℃ |
| CAS DataBase Reference | 106-99-0(CAS DataBase Reference) |
| IARC | 1 (Vol. Sup 7, 54, 71, 97, 100F) 2012 |
| EPA Substance Registry System | 1,3-Butadiene (106-99-0) |
Description and Uses
1,3-Butadiene is a simple conjugated diene. It is a colourless gas with a mild aromatic or gasoline-like odour and incompatible with phenol, chlorine dioxide, copper, and crotonaldehyde. The gas is heavier than air and may travel along the ground; distant ignition is possible. It is an important industrial chemical used as a monomer in the production of synthetic rubber. Most butadiene is polymerised to produce synthetic rubber. While polybutadiene itself is a very soft, almost liquid, material, polymers prepared from mixtures of butadiene with styrene or acrylonitrile, such as ABS, are both tough and elastic. Styrene–butadiene rubber is the material most commonly used for the production of automobile tyres. Smaller amounts of butadiene are used to make nylon via the intermediate adiponitrile, other synthetic rubber materials such as chloroprene, and the solvent sulpholane. Butadiene is used in the industrial production of cyclododecatriene via a trimerisation reaction.
1,3-Butadiene structure
One major use of 1,3-butadiene has been in the
making of synthetic rubber. Among the types of
synthetic rubber made with 1,3-butadiene are
styrene-butadiene and nitrile-butadiene rubbers.
Cis-polybutadiene is also an extender and substitute
for rubber, and trans-polybutadiene is a
type of rubber with unusual properties.
1,3-Butadiene is also used extensively for
various polymerizations in manufacturing plastics.
It combines with activated olefins in the
Diels-Alder reaction to give hydroaromatic hydrocarbons.
1,3-Butadiene undergoes 1,4 cyclization
with reactants containing sulfur, oxygen,
and nitrogen.
Safety
| Symbol(GHS) |    GHS02,GHS04,GHS08 |
| Signal word | Danger |
| Hazard statements | H220-H280-H340 |
| Precautionary statements | P202-P210-P280-P308+P313-P377-P410+P403 |
| Hazard Codes | F+,T,F,N |
| Risk Statements | 45-46-12-67-65-63-48/20-36/38-11-62-51/53-38 |
| Safety Statements | 53-45-62-46-36/37-26-61-33-16 |
| RIDADR | UN 1010 2.1 |
| WGK Germany | 2 |
| RTECS | EI9275000 |
| F | 4.5-31 |
| Autoignition Temperature | 788 °F |
| Hazard Note | Extremely Flammable/Carcinogen |
| DOT Classification | 2.1 (Flammable gas) |
| HazardClass | 2.1 |
| PackingGroup | II |
| HS Code | 29012410 |
| Hazardous Substances Data | 106-99-0(Hazardous Substances Data) |
| Toxicity | LC50 (inhalation) for mice 270 gm/m3/2-h, rats 285 gm/m3/4-h (quoted, RTECS, 1985). |
| IDLA | 2,000 ppm (10% LEL) |