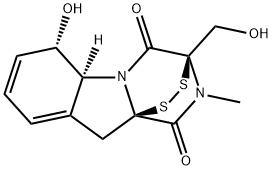
ValproicAcidRelatedCompoundA , PharmaceuticalSecondaryStandard;CertifiedReferenceMaterial , 99-67-2
Synonym(s):
(3R,5aS,6S,10aR)-2,3,5a,6-Tetrahydro-6-hydroxy-3-(hydroxymethyl)-2-methyl-10H-3,10a-epidithiopyrazino[1,2-a]indole-1,4-dione;2,3,5a,6-Tetrahydro-6-hydroxy-3(hyroxymethyl)-2-methyl-10H-3a,10a-epidithio-pyrazinol[1,2α]indole-1,4-dione;Aspergillin;Aspergillin solution;Gliotoxin, Gladiocladium fimbriatum - CAS 67-99-2 - Calbiochem
CAS NO.:99-67-2
Empirical Formula: C13H14N2O4S2
Molecular Weight: 326.39
MDL number: MFCD00058534
EINECS: 636-170-3
| Pack Size | Price | Stock | Quantity |
| 0.5mL | RMB6646.83 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 221°C (rough estimate) |
| Boiling point: | 699.7±55.0 °C(Predicted) |
| alpha | D25 -290° (c = 0.08 in ethanol) |
| Density | 1.4069 (rough estimate) |
| refractive index | 1.6510 (estimate) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | chloroform: 10 mg/mL, clear, colorless |
| form | White solid |
| pka | 12.90±0.40(Predicted) |
| color | Monoclinic crystals from MeOH |
| Merck | 13,4454 |
| BRN | 50675 |
| Stability: | Stable for 2 yeara as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
Description and Uses
Gliotoxin is an immunosuppressive mycotoxin produced by pathogenic strains of Aspergillus and other fungi with diverse biological activities. It inhibits 20S proteasomal chymotrypsin activity (IC50 = 10 μM), blocking the degradation of IκBα and preventing the activation of NF-κB. Gliotoxin induces apoptosis in monocytes and dendritic cells and reduces phagocytosis by neutrophils. It suppresses viral infection by Nipah and Hendra virus in HEK293T cells (IC50s = 149 and 579 nM, respectively). Under reducing conditions, gliotoxin inhibits leukotriene A4 hydrolase (LTA4H; ) epoxide hydrolase activity, but not aminopeptidase activity, and leukotriene B4 (LTB4; ) synthesis in neutrophils and monocytes. In vivo, gliotoxin (5 mg/kg) reduces LTB4 plasma levels and blocks peritoneal neutrophil infiltration in a mouse model of peritonitis induced by zymosan A . It also inhibits geranylgeranyltransferase I and farnesyltransferase (IC50s = 17 and 80 μM, respectively).
Gliotoxin is a potent epithiodioxopiperazine mycotoxin produced by species of Gliocladium, Aspergillus and Penicillium. At the cellular level gliotoxin inhibits a broad range of unrelated mechanisms, including inhibition of chymotrypsin-like activity of the 20S proteasome and Ca2+ release from mitochondria, activation of transcription factor NF-κB in response to a variety of stimuli in T and B cells, anti-inflammatory activity, and inhibition of farnesyltransferase and geranylgeranyltransferase. The mode of action appears to be via covalent interaction with proteins through mixed disulphide formation. Gliotoxin inhibits a number of thiol-requiring enzymes and also displays antioxidant and immunomodulatory activity.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301 |
| Precautionary statements | P301+P310 |
| Hazard Codes | T,Xn,F |
| Risk Statements | 25-36-20/21/22-11 |
| Safety Statements | 36/37/39-45-36/37-16 |
| RIDADR | UN 3462 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | KB4725000 |
| F | 4.2-10-23 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29419090 |
| Toxicity | LD50 oral in mouse: 67mg/kg |