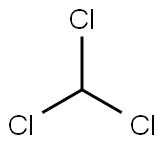
analyticalstandard , 67-66-3
Synonym(s):
Methylidyne trichloride;TCM, Trichloromethane, Methane trichloride, Methyl trichloride;Trichloromethane;Trichloromethane, Chloroform;Trichloromethane, FM approval
CAS NO.:67-66-3
Empirical Formula: CHCl3
Molecular Weight: 119.38
MDL number: MFCD00000826
EINECS: 200-663-8
PRODUCT Properties
| Melting point: | -63 °C |
| Boiling point: | 61 °C |
| Density | 1.492 g/mL at 25 °C(lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 160 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 60.5-61.5°C |
| storage temp. | 2-8°C |
| solubility | Miscible with diethyl ether, oils, ligroin, alcohol, carbon tetrachloride and carbon disulfide. |
| pka | 15,5(at 25℃) |
| form | Liquid |
| color | ≤10(APHA) |
| Relative polarity | 0.259 |
| Odor | Ethereal, sweet odor detectable at 133 to 276 ppm (mean = 192 ppm) |
| Odor Threshold | 3.8ppm |
| Water Solubility | 8 g/L (20 ºC) |
| λmax | λ: 245 nm Amax: 1.00 λ: 255 nm Amax: 0.15 λ: 260 nm Amax: 0.15 λ: 270 nm Amax: 0.02 λ: 290-400 nm Amax: 0.01 |
| Merck | 14,2141 |
| BRN | 1731042 |
| Henry's Law Constant | 1.07 at 2 °C, 1.49 at 6 °C, 1.79 at 10 °C, 3.02 at 18 °C, 4.02 at 25 °C, 5.20 at 30 °C, 7.60 at 40 °C,
11.1 at 50 °C, 14.8 at 60 °C (EPICS-SPME-GC, G?rgényi et al., 2002)) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: STEL (1 h) 2 ppm (9.78
mg/m3), IDLH 500 ppm; OSHA PEL: ceiling 50 ppm (240 mg/m3); ACGIH TLV: TWA 10 ppm
(adopted). |
| Dielectric constant | 5.5(0℃) |
| Stability: | Volatile |
| LogP | 1.970 |
| Surface tension | 27.5mN/m at 20°C |
| CAS DataBase Reference | 67-66-3(CAS DataBase Reference) |
| IARC | 2B (Vol. Sup 7, 73) 1999 |
| NIST Chemistry Reference | Chloroform(67-66-3) |
| EPA Substance Registry System | Chloroform (67-66-3) |
Description and Uses
Chloroform is a trichlorinated organic substance with the chemical formula of CHCl3. It is a clear liquid at room temperature and has a pleasant, sweet odor. Chloroform is only slightly soluble in water and evaporates quickly into surrounding air, increasing the risk of inhalation exposure. In addition, chloroform persists for a long time in both water and air. There are no natural sources of chloroform, but this contaminant enters the environment through a variety of industrial operations, including the chlorination of water. Humans can be exposed to chloroform through inhalation, ingestion, and dermal contact.
Chloroform has a relatively narrow margin of safety and has been replaced by better inhalation anesthetics. In addition, it is believed to be toxic to the liver and kidneys and may cause liver cancer. Chloroform was once widely used as a solvent, but safety and environmental concerns have reduced this use as well. Nevertheless, chloroform has remained an important industrial chemical.
Chloroform is a widely used industrial and laboratory solvent. It is a volatile chlorinated organic solvent whose vapors have a narcotic effect.Due to its light sensitivity, it may undergo degradation with time. This can be suppressed by adding ethanol as a stabilizer. The addition will increase the polarity of the solvent and potentially impact certain applications.
- Chloroform has been used as a solvent for dissolving lipids and also as a cleansing agent.
- It may also be used in solvent extraction process and also for recrystallization.
- Suitable for HPLC, spectrophotometry, environmental testing
- Facilitates recovery of the aqueous phase of PCRs which have been overlaid with mineral oil.
- Meets ACS specifications.
Safety
| Symbol(GHS) |    GHS02,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H225-H319-H336-H351-H361d-H373 |
| Precautionary statements | P202-P210-P233-P240-P305+P351+P338-P308+P313 |
| Hazard Codes | Xn,F,T,Xi |
| Risk Statements | 45-46-11-23/24/25-36/37/38-48/20/22-40-38-22-67-66-36/38-41-37/38-39/23/24/25-20-63-20/22-36-48/20 |
| Safety Statements | 9-16-26-36-36/37-45-36/37/39-25-23-53-33-7 |
| OEL | STEL: 2 ppm (9.78 mg/m3) [60-minute] |
| RIDADR | UN 1888 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | FS9100000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29031300 |
| Hazardous Substances Data | 67-66-3(Hazardous Substances Data) |
| Toxicity | LD50 (14 day) orally in rats: 2.18 ml/kg (Smyth); 0.9 ml/kg (Kimura) |
| IDLA | 500 ppm |