phyproof ReferenceSubstance , 36062-04-1
Synonym(s):
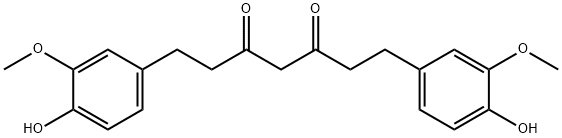
1,7-bis(4-hydroxy-3-methoxyphenyl)-3,5-heptanedione;HZIV 81-2;NSC687845;Tetrahydrocurcumin;Tetrahydrodiferuloylmethane
CAS NO.:36062-04-1
Empirical Formula: C21H24O6
Molecular Weight: 372.41
MDL number: MFCD04152347
EINECS: 609-201-3
| Pack Size | Price | Stock | Quantity |
| 10MG | RMB4488.18 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 95-97 ºC |
| Boiling point: | 564.1±45.0 °C(Predicted) |
| Density | 1.222 |
| Flash point: | 196℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| pka | 9.12±0.10(Predicted) |
| form | powder |
| color | Light Yellow to Yellow |
| BRN | 3485469 |
| Stability: | Light Sensitive |
| InChI | InChI=1S/C21H24O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h5-6,9-12,24-25H,3-4,7-8,13H2,1-2H3 |
| InChIKey | LBTVHXHERHESKG-UHFFFAOYSA-N |
| SMILES | C(C1=CC=C(O)C(OC)=C1)CC(=O)CC(=O)CCC1=CC=C(O)C(OC)=C1 |
| LogP | 2.728 (est) |
Description and Uses
Tetrahydrocurcumin (THC), the active metabolite of curcumin, is gaining popularity amongst scientist due to its wide spectrum of pharmacological activities, better stability and colourless nature. Tetrahydrocurcumin has a similar structure to that of curcumin. THC lacks the α, β-unsaturated carbonyl moiety in its chemical structure. It is naturally sourced from the roots of Curcuma zedoaria, Zingiber mioga, and Zingiber officinale and has potent antioxidant activity and depigmentation of the skin. THC is being studied for the treatment of cancer and dementia. THC is also known to epigenetically ameliorate mitochondrial dysfunction in brain vasculature during Ischemic Stroke. Tetrahydrocurcumin (THC) has been used as a neuroprotective agent to study its therapeutic potential in improving mitochondrial dysfunction in the ischemic stroke mice model.
Tetrahydrocurcumin is an antioxidant and skin-whitening ingredient. It is a major curcuminoid metabolite of curcumin that has been shown to have protective effects against diabetes and vascular dysfunction via alleviation of oxidative stress.The interest in tetrahydrocurcumin research is increasing because it is superior to curcumin in its solubility in water, chemical stability, bioavailability, and anti-oxidative activity. Curcumin metabolizes into tetrahydrocurcumin by bacterial enzyme NADPH-dependent curcumin reductase in the intestine.
Safety
| WGK Germany | 3 |