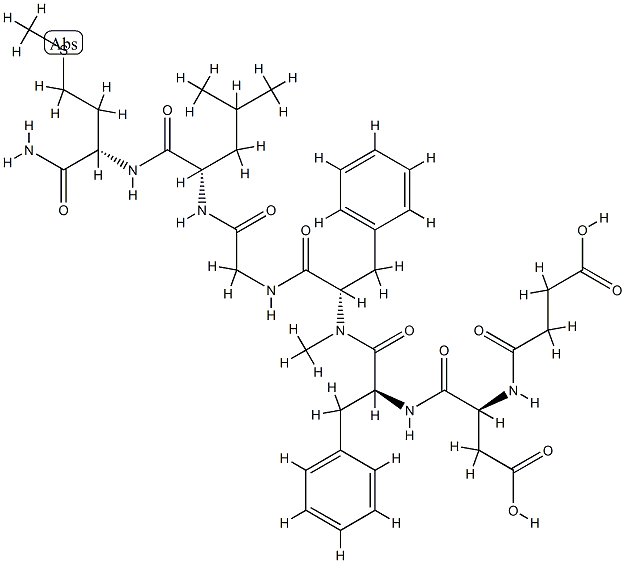
The a4 family of integrins expressed on the surface of leukocytes are involved in cell
adhesion processes. The α4 integrin can pair with either of two b subunits to
generate a heterodimeric cell surface receptor known as α4β1 (VLA4) or α4β7.
Ligands for VLA4 include vascular cell adhesion molecule-1 (VCAM-1), which is
expressed on activated vascular endothelium, while α4β7 interacts predominantly
with mucosal addressin cell adhesion molecule-1 (MadCAM-1) existing on vascular
endothelial cells of the gastrointestinal tract. By virtue of this α4–mediated interaction
between leukocytes and vascular endothelial cells that leads to trans-endothelial
infiltration of various leukocytes (lymphocytes, monocytes, T-cells, etc.) at
the site of inflammation, interference with the adhesion of the α4 integrin has been
deemed a viable approach for disrupting the inflammatory cascade. As an antibody
that binds to the α4 integrin subunit, natalizumab has been developed and launched
for the treatment of multiple sclerosis, a chronic inflammatory disorder of the
central nervous system. It is also being developed for other chronic inflammatory
diseases, such as, Crohn’s disease, rheumatoid arthritis, and irritable bowel syndrome
(IBS). Natalizumab is a recombinant humanized monoclonal antibody produced
in murine myeloma cells. It contains human framework regions and the
complementarity-determining regions of an antibody that is targeted to the α4
integrin. For the treatment of irritable bowel diseases (Crohn’s disease, ulcerative
colitis, and IBS), the target is the α4β7 glycoprotein while efficacy in treating MS is
attributed to binding to the α4 subunit of α4β1. For MS, the binding of natalizumab
prevents docking of VCAM-1 to its receptor on leukocytes, thereby, effectively
inhibiting leukocyte trafficking across the blood brain-barrier (BBB). A
reduction in migration across the BBB translates into a reduction in lesions and
relapses. In a two-year, placebo-controlled, double blind phase III study, a oncemonthly,
300 mg i.v. infusion of natalizumab reduced relapses by 66% compared to
placebo. All of the secondary endpoints, such as, the number of new or newly
enlarging T2-hyperintense lesions, the number of gadolinium-enhancing lesions,
and the proportion of relapse-free patients, were all met. Regarding side effects,
headache, fatigue, and arthralgia were reported in 5% of natalizumab patients, 2% more common than observed with placebo. Serious hypersensitivity-like reactions
were experienced in 1% of the natalizumab group. In these cases, adverse effects
usually developed within two hours of the onset of the infusion. The symptoms
included urticaria, fever, rash, rigors, dizziness, pruritus, nausea, flushing, dyspnea,
hypotension, and chest pain. Antibodies to natalizumab are believed to be responsible,
and any patient experiencing hypersensitivity should discontinue further
treatment. Since adequate studies have not been performed in the pregnant,
pediatric, and elderly, natalizumab is currently contraindicated in these patient
populations. In addition, this drug should not be taken concurrent with medications
that suppress the immune system, such as, corticosteroids; the combination
increases the risk for serious infections. With a dose of 300 mg to MS patients, the
long half-life of 11±4 days results in a once-a-month trip to the physician for the
one-hour infusion. Natalizumab is cleared at a rate of 16 mL/h with a Cmax of 98 μg/
mL and a mean steady-state concentration of approximately 30 μg/mL.
Natalizumab is a recombinant, humanized IgG4 monoclonal antibody, binds to α4β1-integrin and blocks its interaction with vascular cell adhesion molecule-1 (VCAM-1). Natalizumab can be used for the treatment of relapsing remitting multiple sclerosis and Crohn's disease. Natalizumab is also the first targeted therapy which blocks an essential mechanism for lymphocyte entry to the CNS and thus prevents acute demyelinating relapses[1].