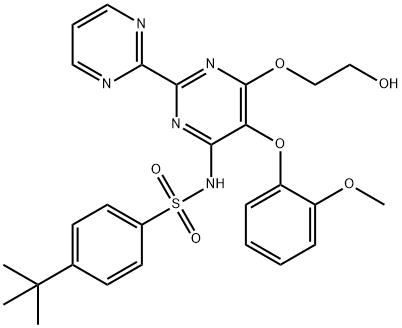
Bosentan? , ≥99% , 147536-97-8
Synonym(s):
4-tert-Butyl-N-[6-(2-hydroxyethoxy)- 5-(2-methoxyphenoxy)-2-(pyrimidin-2-yl)pyrimidin-4-yl]benzenesulfonamide;Bosentan monohydrate
CAS NO.:147536-97-8
Empirical Formula: C27H29N5O6S
Molecular Weight: 551.61
MDL number: MFCD00867375
EINECS: 643-099-1
| Pack Size | Price | Stock | Quantity |
| 50mg | RMB624.80 | In Stock |
|
| 100mg | RMB784.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 107-110°C |
| Boiling point: | 742.3±70.0 °C(Predicted) |
| Density | 1.325±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.01±0.10(Predicted) |
| color | White to Pale Yelloow |
| CAS DataBase Reference | 147536-97-8(CAS DataBase Reference) |
Description and Uses
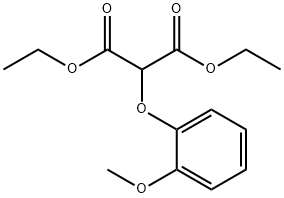
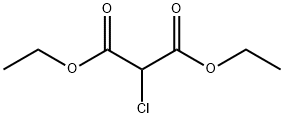
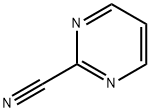
Bosentan was introduced in the US as a twice-daily oral treatment for pulmonary arterial hypertension. It can be synthesized in five steps via condensation of diethyl (2- methoxyphenoxy)malonate with pyrimidine-2-carboxamidine to give the precursor of the symmetrical central dichloropyrimidine ring which is then successively treated with the potassium salt of 4-tert-butylbenzenesulfonamide and the sodium salt of ethylene gycol. Bosentan is the first endothelin (ET) receptor antagonist to be launched. ET-1, the most potent endogenous vasoconstrictor known, has been demonstrated to play a major role in the functional and structural changes observed in pulmonary hypertension. Bosentan is a mixed ETA and ETB receptor antagonist that inhibits the pulmonary arterial vasoconstricting effect of ET-1 predominantly mediated via ETA receptors on smooth muscle cells. In a hypoxia-induced model of pulmonary hypertension in rat, it reduced the development of pulmonary hypertension as well as right ventricular hypertrophy and prevented pulmonary arterial remodeling. In clinical trials, patients treated with bosentan showed a 20% increase in exercise capacity compared to placebo as measured by the six minute walk test. Bosentan not only improved the distance walked by patients but also significantly decreased mean pulmonary artery pressure, mean pulmonary vascular resistance, mean capillary wedge pressure and mean right atrial pressure. It demonstrated a beneficial selectivity for the pulmonary vasculature since it had no significant effect on mean aortic blood pressure and systolic vascular resistance. The compound is hepatically metabolized into three major metabolites by CYP3A4 and 2C9 and almost exclusively eliminated in the bile. Although large interspecies differences in systemic plasma clearance was observed (1.5 mL/min/kg in dogs to 72 mL/min/kg in rabbits), a satisfactory systemic clearance (2 mL/min/kg) was measured in human. The most frequent adverse effect was reversible elevation of liver transaminases. This adverse reaction appears to be due to intracellular accumulation of cytotoxic bile salts resulting from inhibition of the hepatocanalicular bile salt export pump by bosentan.
Bosentan is a mixed endothelin receptor antagonist. Used as a vasodilator. Antihypertensive.
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H302-H360-H410 |
| Precautionary statements | P273-P281 |
| HS Code | 29350090 |

![5-(2-Methoxyphenoxy)-[2,2'-bipyrimidine]-4,6[1H,5H]-dione](https://img.chemicalbook.com/CAS/GIF/150728-12-4.gif)