Sacubitril is a neprilysin
inhibitor prodrug developed by Novartis that was approved
as part of an orally administered supramolecular sodium salt
complex with the angiotensin receptor blocker (ARB) valsartan
in the U.S. and EU in 2015. Sacubitril/valsartan (also known
as LCZ-696) is a first-in-class dual angiotensin receptor
blocker neprilysin inhibitor (ARNI) marketed for the treatment
of chronic heart failure with reduced ejection fraction
(HFrEF). It represents a novel mechanistic approach to
targeting HFrEF and is the first pharmacologic agent approved
for HFrEF since 2004. Sacubitril is metabolized by enzymatic
conversion of the ethyl ester to the active diacid (LBQ-657,
structure not disclosed), which inhibits neprilysin and prevents endogenous natriuretic peptide degradation. Neprilysin
inhibitors like sacubitril are not effective as monotherapy and
need to be combined with a reninangiotensin aldosterone
system (RAAS) inhibitor such as valsartan. Notably, dual
neprilysin and angiotensin-converting enzyme (ACE) inhibition,
as in omapatrilat, was found to be associated with an
increased risk of life-threatening angioedema due to increased
bradykinin levels. In phase III clinical trials, sacubitril/
valsartan displayed a superior safety profile to enalapril, with
a 20% decrease in heart failure hospitalizations or cardiovascular
death and a 16% reduction in the risk of death from any
cause. Sacubitril/valsartan is now recommended as the standard
of care for HFrEF as an alternative to ACEs and ARBs.

![(S)-5-[(Biphenyl-4-yl)methyl]pyrrolidin-2-one](https://img.chemicalbook.com/CAS/20150408/GIF/1038924-61-6.gif)
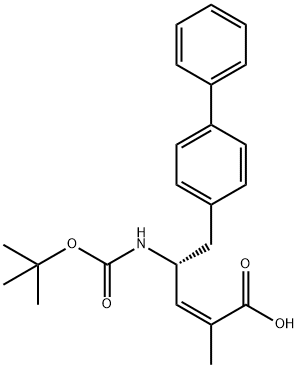
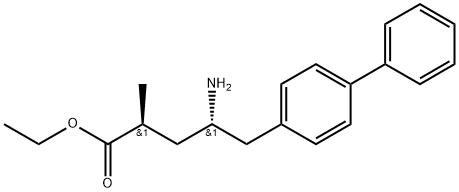
![(R)-tert-Butyl (1-([1,1'-biphenyl]-4-yl)-3-hydroxypropan-2-yl)carbamate](https://img.chemicalbook.com/CAS/20150408/GIF/1426129-50-1.gif)
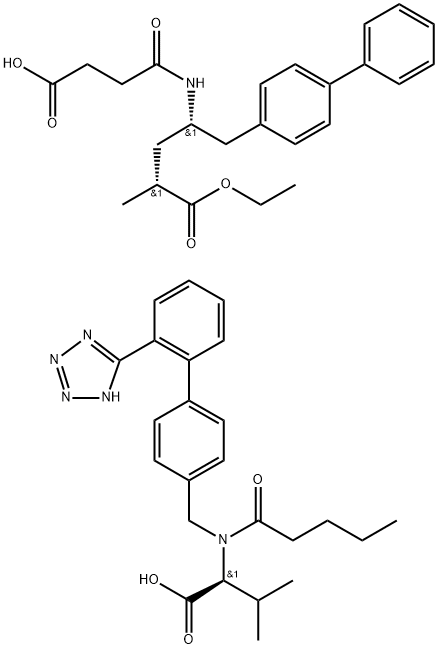
![Ethyl (2R,4S)-4-([1,1'-biphenyl]-4-ylmethyl)-2-methyl-4-(2,5-dioxopyrrolidin-1-yl)butanoate](https://img.chemicalbook.com/CAS/20180703/GIF/1038924-97-8.gif)