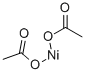
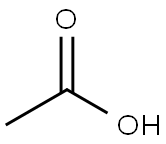
PRODUCT Properties
| Melting point: | 1555°C |
| Boiling point: | 16.6°C |
| Density | 1.798 g/cm3 |
| form | solid |
| Water Solubility | 177g/L at 20℃ |
| EPA Substance Registry System | Nickel(II) acetate (373-02-4) |
Description and Uses
Nickel (II) acetate (Ni (CH3 COO)2 ) is an inorganic compound of nickel and acetic acid. This inorganic compound is usually found as the tetrahydrate. It is used for electroplating.
It can be made by reacting nickel or nickel (II) carbonate with acetic acid.
Ni + 2 CH3 COOH → C4 H6 NiO4 + H2
NiCO3 + 2 CH3 COOH → C4 H6 NiO4 + CO2 + H2 O
The green tetrahydrate has been determined by X-ray crystallography to be octahedral about the central nickel atom, coordinated by four water molecules and two acetate fragments.It may be dehydrated in vacuo, by reaction with acetic anhydride,or by heat.
Textiles (mordant), catalyst.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazardous Substances Data | 373-02-4(Hazardous Substances Data) |
| Toxicity | guinea pig,LDLo,subcutaneous,20mg/kg (20mg/kg),PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE)LUNGS, THORAX, OR RESPIRATION: DYSPNEALUNGS, THORAX, OR RESPIRATION: OTHER CHANGES,Journal of Hygiene. Vol. 8, Pg. 565, 1908. |