PRODUCT Properties
| Melting point: | 1184 °C |
| Density | 503.3[at 20℃] |
| solubility | soluble in dilute acid solutions |
| color | white crystals, crystalline |
| Odor | at 100.00?%. odorless |
| Water Solubility | insoluble H2O; soluble dilute mineral acids [MER06] |
| LogP | -2.148 (est) |
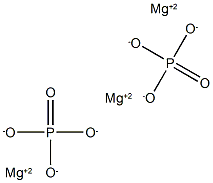
| CAS DataBase Reference | 7757-87-1 |
| EPA Substance Registry System | Trimagnesium phosphate (7757-87-1) |
Description and Uses
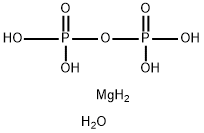
Magnesium phosphate includes both magnesium phosphate, dibasic, and magnesium phosphate, tribasic. Magnesium phosphate, dibasic (MgHPO4.3H2O, CAS Reg. No. 7782-75-4) occurs naturally as the white, crystalline mineral newberyite. It is prepared commercially as a precipitate formed by treating a solution of magnesium sulfate with disodium phosphate under controlled conditions. Magnesium phosphate, tribasic (Mg3(PO4)2.xH2O, CAS Reg. No. 7757-87-1) may contain 4, 5, or 8 molecules of water of hydration. It is produced as a precipitate from a solution of magnesite with phosphoric acid. Magnesium plays a vital role in regulating the neuromuscular activity of the heart, converts blood sugar to energy and is necessary for proper calcium and Vitamin C metabolism. Magnesium Phosphate can be used as a dietary supplement and as a nutrient.
Magnesium Phosphate (Mag Phos) is the painkiller of the cell salts. It is taken against pains and cramps. It is the eighth of the twelve cell salts and has been called the "homeopathic aspirin" because of its ability to heal acute pains anywhere such as earache, headache, toothache (including teething in babies), and even sciatica—as long as the pains are better with the use of heat and pressure. It is often used as a hot drink. For this drink dissolve ten tablets of Magnesium phosphate in hot water an drink in small sips. the cell salt Magnesium phosphate is assimilated especially fast by the body and thereby works quite good. This mixture may not be stirred with metal spoons.
Safety
| HS Code | 2835299050 |
| Hazardous Substances Data | 7757-87-1(Hazardous Substances Data) |