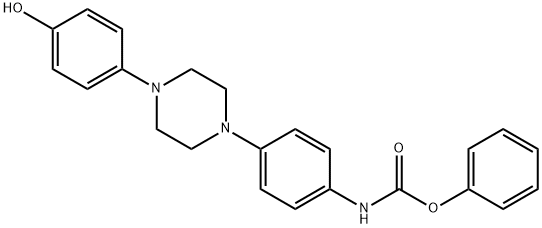
Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate , 97% , 184177-81-9
| Pack Size | Price | Stock | Quantity |
| 1g | RMB24.00 | In Stock |
|
| 5g | RMB28.80 | In Stock |
|
| 25g | RMB118.40 | In Stock |
|
| 100g | RMB428.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Boiling point: | 603.9±55.0 °C(Predicted) |
| Density | 1.296 |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 12.18±0.30(Predicted) |
| InChI | InChI=1S/C23H23N3O3/c27-21-12-10-20(11-13-21)26-16-14-25(15-17-26)19-8-6-18(7-9-19)24-23(28)29-22-4-2-1-3-5-22/h1-13,27H,14-17H2,(H,24,28) |
| InChIKey | IKRKMYDCUZYKHX-UHFFFAOYSA-N |
| SMILES | C(OC1=CC=CC=C1)(=O)NC1=CC=C(N2CCN(C3=CC=C(O)C=C3)CC2)C=C1 |
| CAS DataBase Reference | 184177-81-9 |
Description and Uses
Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate is a piperazine derivative that inhibits the growth of gram-positive bacteria.Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)phenyl)carbamate is a carbamic acid that binds to the 50S ribosomal subunit of bacteria, preventing transcription and replication. Phenyl (4-(4-(4-hydroxyphenyl)piperazin-1-yl)) carbamate is a carbamic acid that binds to the 50S ribosomal subunit of bacteria, preventing transcription and replication. Human activity on human erythrocytes using the membrane clamp technique is frequent. This active form is metabolised by a number of metabolic transformations including hydrolysis by esterases or glucuronidases, oxidation by cytochrome P450 enzymes, reduction by glutathione reductase, or binding to glucuronic acid.
[4-[4-(4-Hydroxyphenyl)-1-piperazinyl]phenyl]carbamic Acid Phenyl Ester is an impurity of Posaconazole (P689600), which is orally active antifungal triazole.
Safety
| Symbol(GHS) |  GHS09 |
| Signal word | Warning |
| Hazard statements | H410 |
| Precautionary statements | P273-P391-P501 |