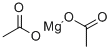PRODUCT Properties
| Melting point: | 72-75 °C(lit.) |
| Density | 1.5000 |
| refractive index | n |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | 4.756[at 20 ℃] |
| form | Liquid |
| color | white orthorhombic/monoclinic
crystals, crystalline |
| PH | 7.95(1 mM solution);8.23(10 mM solution);8.41(100 mM solution);8.78(1000 mM solution) |
| Water Solubility | Not miscible or difficult to mix with water. |
| λmax | λ: 260 nm Amax: 0.04 λ: 280 nm Amax: 0.02 |
| Merck | 13,5676 |
| BRN | 3692530 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Avoid moisture. |
| InChIKey | UEGPKNKPLBYCNK-UHFFFAOYSA-L |
| LogP | -1.38 |
| CAS DataBase Reference | 142-72-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium acetate(142-72-3) |
| EPA Substance Registry System | Magnesium acetate (142-72-3) |
Description and Uses
Anhydrous Magnesium Acetate has the chemical formula Mg(CH3COO)2 and in its hydrous form, Magnesium Acetate Tetrahydrate, it has the chemical formula Mg(CH3COO)2.4H2O. In this compound the magnesium metal has an oxidation state of 2+.
Magnesium acetate is the magnesium salt of acetic acid.It is deliquescent and upon heating, it decomposes to form magnesium oxide.Magnesium acetate is commonly used as a source of magnesium or as a chemical reagent.
Magnesium acetate is used in molecular biology applications such as batch in vitro transcription of RNA and the crystallization of transcription factor:DNA complexes and proteins via the sitting-drop vapor-diffusion method. Also used as a buffer and to detect sodium and as a catalyst for sulfuric acid.
Safety
| Symbol(GHS) |   GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H312-H226-H332 |
| Precautionary statements | P280-P302+P352-P312-P322-P363-P501-P261-P271-P304+P340-P312 |
| Safety Statements | 22-24/25 |
| WGK Germany | 3 |
| RTECS | AI5600000 |
| F | 3 |
| TSCA | Yes |