RamosetronHydrochloride , 98% , 132907-72-3
Synonym(s):
(R)-5-[(1-Methyl-3-indolyl)carbonyl]-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride;YM060;YM-060
| Pack Size | Price | Stock | Quantity |
| 100mg | RMB201.60 | In Stock |
|
| 250mg | RMB342.40 | In Stock |
|
| 1g | RMB923.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 244-246°C |
| alpha | D -42.9° (c 1.02 in methanol) |
| storage temp. | -20°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -40 to -45°, c = 1 in methanol |
| Water Solubility | H2O: 20mg/mL, clear |
| Stability: | Hygroscopic |
| InChIKey | XIXYTCLDXQRHJO-RFVHGSKJSA-N |
| CAS DataBase Reference | 132907-72-3(CAS DataBase Reference) |
Description and Uses
Ramosetron Hydrochloride is the only currently available drug indicated for the treatment of male patients with IBS-D in Japan. Ramosetron Hydrochloride was approved in July 1996 under a brand name of Nasea Injection 0.3 mg and in June 1998 under a brand name of Nasea OD tablets 0.1 mg for the treatment of gastrointestinal symptoms (nausea and vomiting) associated with therapy with antineoplastic drugs (e.g., cisplatin).
Nasea was launched in Japan for chemotherapy-induced emesis. Nasea was prepared by a four step sequence via the Vilsmeier-type coupling of 1-methylindole and 5-( 1- pyrrolidoncarbonyl)-4,5,6,7-tetrahydro-Hl-benzimidazole hydrochloride. The antiemetic activity arises because it is a potent 5-HT3 receptor antagonist that is i.v. and orally active. The (R)-isomer was found to be 100 times more potent than the (S)-isomer. It was able to inhibit cisplatin-induce emesis and was 8670 times more potent than netoclopramide.
Ramosetron Hydrochloride is a selective serotonin 5-HT3 receptor antagonist and has structurally different from Ondansetron (O655000), Granisetron (G780000). It controls excessive bowel movement and diarrhea by inhibiting 5-HT3 receptors in the intestinal tract. Ramosetron Hydrochloride was approved as film-coated tablets in July 2008 and as orally disintegrating tablets in August 2013 for the indication of treatment of male patients with diarrhea-predominant irritable bowel syndrome.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264; P270; P301+P312; P330; P501 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| WGK Germany | 3 |
| Toxicity | dog,LD,intravenous,> 30mg/kg (30mg/kg),SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE,Oyo Yakuri. Pharmacometrics. Vol. 47, Pg. 117, 1994. |

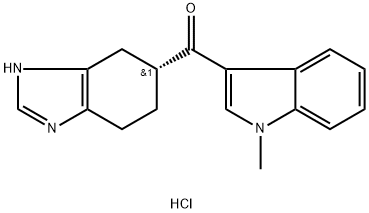
![5-(1-methyl-1H-indole-3-carbonyl)-1H-benzo[d]imidazol-2(3H)-one](https://img.chemicalbook.com/CAS/GIF/171967-71-8.gif)
![4,5,6,7-Tetrahydro-1H-benzo[d]imidazole-5-carboxylic acid hydrochloride](https://img.chemicalbook.com/CAS/GIF/131020-57-0.gif)