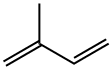
Isoprene , > 99.0%(GC), containing 200 ppm4-olinchide dilate stabilizer , 78-79-5
Synonym(s):
2-Methyl-1,3-butadiene;Isoprene
CAS NO.:78-79-5
Empirical Formula: C5H8
Molecular Weight: 68.12
MDL number: MFCD00008600
EINECS: 201-143-3
PRODUCT Properties
| Melting point: | 323-329 °C(lit.) |
| Boiling point: | 34 °C(lit.) |
| Density | 0.681 g/mL at 25 °C(lit.) |
| vapor density | 2.35 (vs air) |
| vapor pressure | 8.82 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −65 °F |
| storage temp. | Store at <= 20°C. |
| solubility | 0.7g/l |
| form | solid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Clear colorless to very pale yellow |
| Odor | petroleum-like odor |
| Odor Threshold | 0.048ppm |
| explosive limit | 1-9.7%(V) |
| Water Solubility | 0.07 g/100 mL |
| FreezingPoint | -145.96℃ |
| λmax | 231nm(neat)(lit.) |
| Merck | 14,5201 |
| BRN | 969158 |
| Henry's Law Constant | (x 10-2 atm?m3/mol):
3.45 at 18 °C (dynamic stripping cell-MS, Karl et al., 2003) |
| Dielectric constant | 2.1(25℃) |
| Stability: | Stability Extremely flammable. Readily forms explosive mixtures with air. Note low flash point, low boiling point, high vapour pressure. Unstable - prone to spontaneous polymerization. May contain a polymerization inhibitor. Incompatible with strong oxidizing agents. |
| LogP | 2.42 at 20℃ |
| CAS DataBase Reference | 78-79-5(CAS DataBase Reference) |
| IARC | 2B (Vol. 60, 71) 1999 |
| NIST Chemistry Reference | 1,3-Butadiene, 2-methyl-(78-79-5) |
| EPA Substance Registry System | Isoprene (78-79-5) |
Description and Uses
Isoprene is a naturally occurring clear colorless volatile liquid
(at room temperature) with a faint odor. Isoprene is an
important building block for lipids, steroids, terpenoids, and
a wide variety of natural products, including natural rubber.
Isoprene is found in abundance in nature and is produced and
emitted to the environment by plants and trees; it is also
emitted from food crops since isoprene serves as the basic
structural unit of numerous substances such as terpenes and
vitamins A and K. Hence, isoprene is found in ambient air at
low concentrations (e.g., the reported concentration of
isoprene in the ambient air of the United States ranges
between 1 and 21 ppb and is generally less than 10 ppb).
Because the biosynthesis of isoprene is associated with
photosynthesis, emission of isoprene from plants and trees is
negligible at night. Emission of isoprene from plants and trees
is also seasonal with the highest emission occurring in the
summer and the lowest emission occurring in the winter.
Once emitted to the atmosphere, isoprene is converted by free
radicals (e.g., nitric oxide, hydroxyl radicals, ozone) to various
species (e.g., aldehydes, hydroperoxides, organic nitrates,
epoxides) that mix with water droplets and help create aerosols
and haze.
Isoprene is produced endogenously in experimental
animals and humans. The rate of endogenous production of
isoprene in rats and mice is approximately 1.9 and
0.4 mmol kg-1 h-1, respectively, while in humans it is approximately
0.15 mmol kg-1 h-1 (approximately 2–4 μmol kg-1 per
day). The precursor to endogenous isoprene production in
humans is believed to be mevalonic acid, a precursor of
cholesterol synthesis. Isoprene is the major hydrocarbon found
in human breath, accounting for up to 70% of exhaled
hydrocarbons. The mean concentration of isoprene reported
in human breath is 118 ppb (range 0–474 ppb). The concentration
of isoprene in human blood is between 1 and
4.8 μg l-1. The rate of production of isoprene is higher in males
than females and in adults than in children.
Anthropogenic sources of isoprene include production of
ethylene by cracking naphtha, wood pulping, combustion of
wood and other biomass, tobacco smoke (smoking one cigarette
can increase the concentration of isoprene in exhaled air
by 70%), gasoline, and automobile exhaust.
The majority of isoprene produced commercially is used to make synthetic rubber (cis-polyisoprene), most of which is used to produce vehicle tires. The second- and third-largest uses are in the production of styrene-isoprene-styrene block polymers and butyl rubber (isobutene-isoprene copolymer) (IARC 1994).
Safety
| Symbol(GHS) |   GHS02,GHS08 |
| Signal word | Danger |
| Hazard statements | H224-H341-H350-H412 |
| Precautionary statements | P202-P210-P233-P273-P308+P313-P403+P233 |
| Hazard Codes | F+,T,N |
| Risk Statements | 45-12-52/53-68-51/53 |
| Safety Statements | 53-45-61-36/37-16 |
| RIDADR | UN 1218 3/PG 1 |
| WGK Germany | 1 |
| RTECS | NT4037000 |
| Autoignition Temperature | 428 °F |
| TSCA | Yes |
| HS Code | 2901 24 00 |
| HazardClass | 3 |
| PackingGroup | I |
| Hazardous Substances Data | 78-79-5(Hazardous Substances Data) |
| Toxicity | LD50 for mice: 144 mg isoprene vapors/l air (Gostinskii) |