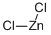Zinc chloride , 99.999%metalsbasis , 7646-85-7
Synonym(s):
Additive Screening Solution 29/Kit-No 78374;Dichlorozinc;Zinc chloride;Zinc chloride solution;Zinc dichloride
CAS NO.:7646-85-7
Empirical Formula: Cl2Zn
Molecular Weight: 136.3
MDL number: MFCD00004679
EINECS: 231-592-0
PRODUCT Properties
| Melting point: | 293 °C (lit.) |
||||||||||||||
| Boiling point: | 732 °C (lit.) |
||||||||||||||
| Density | 1.01 g/mL at 20 °C |
||||||||||||||
| bulk density | 1400-1800kg/m3 |
||||||||||||||
| vapor pressure | 1 mm Hg ( 428 °C) |
||||||||||||||
| Flash point: | 732°C |
||||||||||||||
| storage temp. | 2-8°C |
||||||||||||||
| solubility | H2O: 4 M at 20 °C, clear, colorless |
||||||||||||||
| pka | pKa 6.06 (Uncertain) |
||||||||||||||
| form | crystalline |
||||||||||||||
| Specific Gravity | 2.91 |
||||||||||||||
| color | white |
||||||||||||||
| PH | 5 (100g/l, H2O, 20℃) |
||||||||||||||
| Odor | wh. cubic cryst., odorless |
||||||||||||||
| Water Solubility | 432 g/100 mL (25 ºC) |
||||||||||||||
| Sensitive | Hygroscopic |
||||||||||||||
| Merck | 14,10132 |
||||||||||||||
| crystal system | square |
||||||||||||||
| Space group | I42d |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 1 mg/m3; STEL 2 mg/m3 OSHA: TWA 1 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 1 mg/m3; STEL 2 mg/m3 |
||||||||||||||
| Stability: | hygroscopic |
||||||||||||||
| InChIKey | JIAARYAFYJHUJI-UHFFFAOYSA-L |
||||||||||||||
| CAS DataBase Reference | 7646-85-7(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Zinc dichloride(7646-85-7) |
||||||||||||||
| EPA Substance Registry System | Zinc chloride (7646-85-7) |
Description and Uses
Zinc chloride is a white deliquescent salt. It forms acidic solutions in water and in polar organic solvents such as ethanol, acetone, and ether. Anhydrous zinc chloride hydrolyzes with moisture to form hydrochloric acid. It also forms complex ions with water, ammonia, and some organic solvents. Zinc chloride reacts with sulphide to minimise release of H2S gas in waste treatment facilities. Zinc chloride 50% solution also serves as a high-quality mercerising agent for cotton. Zinc chloride is incompatible with strong oxidising agents, moisture, cyanides, sulphides, and potassium.
Zinc chloride is used as an organic catalyst. It is deliquescent, which makes it an excellent dehydrating and drying agent. It is used in electroplating other metals, as an antiseptic, as a component of some deodorants, and as an astringent. It is also used for fireproofing materials and as a food preservative. Zinc chloride is also used in embalming and taxidermy fluids.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
| Signal word | Danger |
| Hazard statements | H302-H314-H410 |
| Precautionary statements | P260-P273-P280-P301+P312-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | Xi,N,C,F+,F,Xn |
| Risk Statements | 36/37/38-50/53-34-22-51/53-67-66-19-12-11-40 |
| Safety Statements | 26-36-61-60-45-36/37/39-16-36/37 |
| OEB | C |
| OEL | TWA: 1 mg/m3, STEL: 2 mg/m3 |
| RIDADR | UN 2924 3/PG 1 |
| WGK Germany | 2 |
| RTECS | TY2900000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | I |
| HS Code | 28273600 |
| Hazardous Substances Data | 7646-85-7(Hazardous Substances Data) |
| Toxicity | Inhalation of zinc chloride fumes can injure lungs and respiratory tract. Dusts or fumes also cause dermatitis, boils, conjunctivitis, and gastrointestinal tract upset (Lewis(Sr), R.J. 1996. Sax’s Dangerous Properties of Industrial Materials, 9th ed. New York: Van Nostrand Reinhold). LD50 oral (rat): 350mg/kg LCLO (inhalation): 1.960 g/m3/10 min |
| IDLA | 50 mg/m3 |