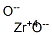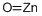Zirconium dioxide , 99.99%metalsbasis (remove HFORHFO2) , 1314-23-4
Synonym(s):
Zirconia
CAS NO.:1314-23-4
Empirical Formula: O2Zr
Molecular Weight: 123.22
MDL number: MFCD00011310
EINECS: 215-227-2
| Pack Size | Price | Stock | Quantity |
| 25G | RMB28.80 | In Stock |
|
| 100G | RMB60.80 | In Stock |
|
| 500G | RMB255.20 | In Stock |
|
| 2.5KG | RMB984.80 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 2700 °C(lit.) |
||||||||||||||
| Boiling point: | 5000 °C(lit.) |
||||||||||||||
| Density | 5.89 g/mL at 25 °C(lit.) |
||||||||||||||
| Flash point: | 5000°C |
||||||||||||||
| solubility | insoluble |
||||||||||||||
| form | powder |
||||||||||||||
| Specific Gravity | 5.89 |
||||||||||||||
| color | White |
||||||||||||||
| PH | 4-5 |
||||||||||||||
| Resistivity | 2.3 × 10*10 (ρ/μΩ.cm) |
||||||||||||||
| Water Solubility | insoluble |
||||||||||||||
| Crystal Structure | Monoclinic; Tetragonal; Cubic |
||||||||||||||
| crystal system | Monoclinic |
||||||||||||||
| Merck | 14,10180 |
||||||||||||||
| Space group | P21/c |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 12.5(0.0℃) |
||||||||||||||
| Exposure limits | ACGIH: TWA 5 mg/m3; STEL 10 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 5 mg/m3; STEL 10 mg/m3 |
||||||||||||||
| Stability: | Stable. |
||||||||||||||
| InChIKey | RVTZCBVAJQQJTK-UHFFFAOYSA-N |
||||||||||||||
| CAS DataBase Reference | 1314-23-4(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Zirconium dioxide(1314-23-4) |
||||||||||||||
| EPA Substance Registry System | Zirconium oxide (1314-23-4) |
Description and Uses
Zirconium oxide (ZrO2) is the most common compound of zirconium found in nature. It has many uses, including the production of heat-resistant fabrics and high-temperature electrodes and tools, as well as in the treatment of skin diseases. The mineral baddeleyite (known as zirconia or ZrO2) is the natural form of zirconium oxide and is used to produce metallic zirconium by the use of the Kroll process. The Kroll process is used to produce titanium metal as well as zirconium. The metals, in the form of metallic tetrachlorides, are reduced with magnesium metal and then heated to “red-hot” under normal pressure in the presence of a blanket of inert gas such as helium or argon.
Safety
| Symbol(GHS) |    GHS05,GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H318-H222-H229-H319-H335-H314 |
| Precautionary statements | P264-P280a-P305+P351+P338-P310a-P321-P332+P313-P210-P211-P251-P280i-P410+P412-P260h-P301+P330+P331-P303+P361+P353-P405-P501a-P261-P304+P340 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37-39-36 |
| HS Code | 28256000 |