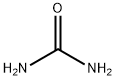
Urea , AR,99% , 57-13-6
Synonym(s):
Urea;Carbamide;NBK;Urea solution;Ureum
CAS NO.:57-13-6
Empirical Formula: CH4N2O
Molecular Weight: 60.06
MDL number: MFCD00008022
EINECS: 200-315-5
| Pack Size | Price | Stock | Quantity |
| 500G | RMB39.20 | In Stock |
|
| 2.5KG | RMB159.20 | In Stock |
|
| 12×500g | RMB479.20 | In Stock |
|
| 20×500g | RMB603.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 132-135 °C(lit.) |
| Boiling point: | 332.48°C (estimate) |
| Density | 1.335 g/mL at 25 °C(lit.) |
| bulk density | 720-760kg/m3 |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | n |
| storage temp. | 2-8°C |
| solubility | H2O: 8 M at 20 °C |
| form | powder |
| pka | 0.10(at 25℃) |
| color | white |
| Specific Gravity | 1.335 |
| Odor | almost odorless |
| PH | 8.0-10.0 (20℃, 8M in H2O) |
| biological source | synthetic |
| Water Solubility | 1080 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,9867 |
| BRN | 635724 |
| Dielectric constant | 3.5(Ambient) |
| Stability: | Substances to be avoided include strong oxidizing agents. Protect from moisture. |
| InChIKey | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
| LogP | -1.660 (est) |
| CAS DataBase Reference | 57-13-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Urea(57-13-6) |
| EPA Substance Registry System | Urea (57-13-6) |
| Absorption | ≤0.06 at 260nm at 5M ≤0.06 at 280nm at 5M |
Description and Uses
Urea is a stable highly water-soluble compound
of high nitrogen content (47%), with good storage
properties that make it the most commonly used nitrogen
fertilizer. The synthesis process has remained essentially
unchanged since it was first developed by the BASF
Corporation in 1922. In this process, liquid ammonia
is reacted with carbon dioxide to produce ammonium
carbamate, which is then dehydrated to form urea. The
reactions are:
2NH3 + CO2 ===? NH2·CO2·NH4
NH2·CO2·NH4 ===? (NH2)2CO + H2O
The primary use of urea is as a nitrogen source in fertilizers, with about 90% of the urea production being used for this purpose. Urea's high nitrogen content (46%) makes it a concentrated source for adding fixed nitrogen to soils. It can be applied to the soil alone, but its high nitrogen content can stress plants and impact the soil negatively, so it is often blended with other nutrients. Blending also reduces the nitrogen content of the fertilizer. For example, blending with ammonium nitrate, NH4NO3, in different proportions produces fertilizers with various nitrogen contents. Urea in the soil is converted to ammonium nitrogen and taken up by plants. It can be applied in solid granule form or dissolved in water and used as a spray. Urea is also used agriculturally as a supplement in livestock feeds to assist protein synthesis.
Another use of urea is for resins, which are used in numerous applications including plastics, adhesives, moldings, laminates, plywood, particleboard, textiles, and coatings. Resins are organic liquid substances exuded from plants that harden on exposure to air. The term now includes numerous synthetically produced resins. Urea resins are thermosetting, which means they harden when heated, often with the aid of a catalyst. The polymerization of urea and formaldehyde produces urea-formaldehyde resins, which is the second most abundant use of urea. Urea is dehydrated to melamine, which, when combined with formaldehyde, produces melamine-formaldehyde resins.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H320 |
| Precautionary statements | P264-P337+P313-P305+P351+P338 |
| Hazard Codes | Xn,Xi |
| Risk Statements | 36/37/38-40-38 |
| Safety Statements | 26-36-24/25-37 |
| RIDADR | Not regulated |
| WGK Germany | 1 |
| RTECS | YR6250000 |
| TSCA | Yes |
| HS Code | 31021010 |
| Hazardous Substances Data | 57-13-6(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 8471 mg/kg LD50 dermal Rat 8200 mg/kg |