A7946012
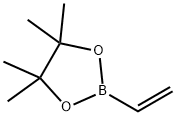
4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane (stabilized with Phenothiazine) , ≥93.0% , 75927-49-0
Synonym(s):
2-Ethenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane;2-Vinyl-4,4,5,5-tetramethyl-1,3,2-dioxaoborolane;4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane
| Pack Size | Price | Stock | Quantity |
| 1G | RMB31.20 | In Stock |
|
| 5G | RMB86.40 | In Stock |
|
| 25G | RMB317.60 | In Stock |
|
| 100g | RMB959.20 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Boiling point: | 52-54°C 24mm |
| Density | 0.908 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 94 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly) |
| form | Powder or Low Melting Solid |
| color | White to yellow |
| Sensitive | Air Sensitive |
| Stability: | Light Sensitive, Volatile |
| CAS DataBase Reference | 75927-49-0(CAS DataBase Reference) |
Description and Uses
Reagent used for
- Suzuki-Miyaura coupling reactions
- Mizoroki-Heck reactions (cascade reaction)
- Intramolecular Nozaki-Hiyama-Kishi reactions
- Stereoselective Cu-catalyzed γ-selective and stereospecific coupling
- Control of stereoselectivity and mechanistic portrait on intramolecular (4+1)-cycloaddition of dialkoxycarbenes
- Regio- and stereoselective synthesis of trisubstituted alkenes via gold(I)-catalyzed hydrophosphoryloxylation of haloalkynes followed by Pd-catalyzed consecutive cross-coupling reactions
- Asymmetric Birch reductive alkylation
Reagent used in Preparation of
- Molecular tubes for lipid sensing
- Enzymatic inhibitors, antibiotics, receptor analogs, and other biologically significant compounds (including total syntheses)
Safety
| Symbol(GHS) |    GHS02,GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H226-H317-H411 |
| Precautionary statements | P210-P233-P240-P273-P280-P303+P361+P353 |
| Hazard Codes | Xi |
| Risk Statements | 10-43 |
| Safety Statements | 16-36/37 |
| RIDADR | UN 3272 3/PG 3 |
| WGK Germany | 3 |
| TSCA | No |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29319090 |