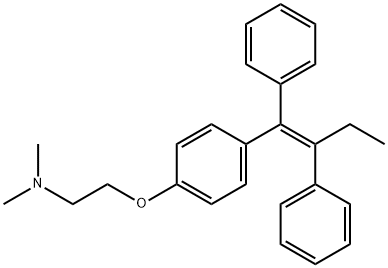
Tamoxifen , Analysis of standard products, ≥99.0%(HPLC) , 10540-29-1
Synonym(s):
(Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene;trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine;Tamoxifen
CAS NO.:10540-29-1
Empirical Formula: C26H29NO
Molecular Weight: 371.51
MDL number: MFCD00010454
EINECS: 234-118-0
| Pack Size | Price | Stock | Quantity |
| 50MG | RMB215.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 97-98 °C(lit.) |
| Boiling point: | 501.18°C (rough estimate) |
| Density | 1.0630 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble <0.1% at 20°C |
| pka | pKa 8.71(H2O
t = 25
I = 0.025) (Uncertain) |
| form | Solid |
| color | Crystals from pet ether |
| Water Solubility | Insoluble in water. Soluble in methanol, ethanol, propanol or propylene glycol.Soluble in dimethyl sulfoxide, dichloromethane and ethanol. Insoluble in water. |
| Merck | 13,9137 |
| Stability: | Light Sensitive |
| InChIKey | NKANXQFJJICGDU-QPLCGJKRSA-N |
| LogP | 6.3 at 20℃ |
| CAS DataBase Reference | 10540-29-1(CAS DataBase Reference) |
| IARC | 1 (Vol. 66, 100A) 2012 |
| EPA Substance Registry System | Tamoxifen (10540-29-1) |
Description and Uses
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized tamoxifen in the hope of developing a morning-after contraceptive pill. The UK patent for this compound was in place in 1962, whereas the US patent was repeatedly denied until the 1980s. Tamoxifen was approved for a fertility treatment but it was not proven as useful in regulating human contraception. Even though there was a link between estrogen and breast cancer, developing a cancer treatment was not a priority at the time. In 1971, the first clinical study showed a convincing effect of tamoxifen in treating advanced breast cancer. From 1971 to 1977, this drug was neither clinically nor financially remarkable. In 1980s, however, publications first showed that tamoxifen, in addition to chemotherapy, improved survival for patients with early stage breast cancer. In 1998, the meta-analysis by the Oxford-based Early Breast Cancer Trialists’ Collaborative Group showed that tamoxifen did indeed save lives in early breast cancer. In 2001, tamoxifen sales were over $1.024 billion. Since the expiration of the patent in 2002, it is now widely available as a generic drug. By 2004, tamoxifen was the best selling hormonal drug for the treatment of breast cancer.
Tamoxifen has been used to facilitate the recombination of ect2flox allele in mouse organs91. It has also been used to study its effect on lipopolysaccharide (LPS)-induced microglial activation92.
Safety
| Symbol(GHS) |   GHS08,GHS09 |
| Signal word | Danger |
| Hazard statements | H350-H360-H410 |
| Precautionary statements | P201-P273-P308+P313 |
| Hazard Codes | T,Xi |
| Risk Statements | 45-60-61-64-36/37/38 |
| Safety Statements | 53-45-36-26 |
| WGK Germany | 3 |
| RTECS | KR5919600 |
| HS Code | 29221990 |
| Hazardous Substances Data | 10540-29-1(Hazardous Substances Data) |
| Toxicity | LD50 orl-rat: 4100 mg/kg DRFUD4 9,186,84 |