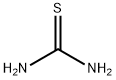
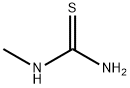
Thiourea , AR,99% , 62-56-6
Synonym(s):
Sulfourea;Thiocarbamide;Thiourea
CAS NO.:62-56-6
Empirical Formula: CH4N2S
Molecular Weight: 76.12
MDL number: MFCD00008067
EINECS: 200-543-5
PRODUCT Properties
| Melting point: | 170-176 °C (lit.) |
| Boiling point: | 263.89°C (estimate) |
| Density | 1.405 |
| bulk density | 640kg/m3 |
| refractive index | 1.5300 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | water: soluble137g/L at 20°C |
| form | Crystals |
| pka | -1.0(at 25℃) |
| Specific Gravity | 1.406 |
| color | White to almost white |
| PH | 6-8 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 5 - 7 |
| Water Solubility | 13.6 g/100 mL (20 ºC) |
| Merck | 14,9367 |
| BRN | 605327 |
| Stability: | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents, metallic salts, proteins, hydrocarbons. May react violently with acrolein. |
| Cosmetics Ingredients Functions | ANTIMICROBIAL HUMECTANT |
| InChIKey | UMGDCJDMYOKAJW-UHFFFAOYSA-N |
| LogP | -1.050 (est) |
| CAS DataBase Reference | 62-56-6(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 79) 2001 |
| NIST Chemistry Reference | Thiourea(62-56-6) |
| EPA Substance Registry System | Thiourea (62-56-6) |
Description and Uses
Thiourea appears as white crystal/powder, is combustible, and on contact with fire, gives off irritating or toxic fumes/gases. Thiourea is a reducing agent used primarily in the production of bleached recycled pulp. In addition, it is also effective in the bleaching of stone groundwood, pressurised groundwood. Thiourea undergoes decomposition on heating and produces toxic fumes of nitrogen oxides and sulphur oxides. It reacts violently with acrolein, strong acids, and strong oxidants. The main application of thiourea is in textile processing and also is commonly employed as a source of sulphide. Thiourea is a precursor to sulphide to produce metal sulphides, for example, mercury sulphide, upon reaction with the metal salt in aqueous solution. The industrial uses of thiourea include production of flame-retardant resins and vulcanisation accelerators. Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper, and almost all other types of copy paper. Thiourea is used in many industrial applications, including as a chemical intermediate or catalyst, in metal processing and plating, and in photoprocessing.
The most common uses for thiourea have been for the production of thiourea dioxide (30%), in leaching of gold and silver ores (25%), in diazo papers (15%), and as a catalyst in the synthesis of fumaric acid (10%) (IARC 2001). It has also been used in the production and modification of synthetic resins. Other uses of thiourea are as a photographic toning agent, in hair preparations, as a drycleaning agent, in the synthesis of pharmaceuticals and pesticides, in boiler-water treatment, and as a reagent for bismuth and selenite ions. It has also been used in textile and dyeing auxiliaries, in the production of industrial cleaning agents (e.g., for photographic tanks and metal surfaces in general), for engraving metal surfaces, as an isomerization catalyst in the conversion of maleic to fumaric acid, in copper-refining electrolysis, in electroplating, and as an antioxidant. Other uses have included as a vulcanization accelerator, an additive for slurry explosives,as a viscosity stabilizer for polymer solutions, and as a mobility buffer in petroleum extraction. It is also used as an ingredient of consumer silver polishes (HPD 2009), and has been used in the removal of mercury from wastewater by chlorine-alkali electrolysis (IARC 1974, 2001, WHO 2003).
Safety
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
| Signal word | Warning |
| Hazard statements | H302-H351-H361fd-H411 |
| Precautionary statements | P201-P202-P264-P273-P301+P312-P308+P313 |
| Hazard Codes | Xn,N,Xi |
| Risk Statements | 22-40-51/53-63-43-38 |
| Safety Statements | 36/37-61 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | YU2800000 |
| Autoignition Temperature | 440 °C Dust |
| TSCA | TSCA listed |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29309070 |
| Hazardous Substances Data | 62-56-6(Hazardous Substances Data) |
| Toxicity | LD50 orally in wild Norway rats: 1830 mg/kg (Dieke) |
| Limited Quantities | 5.0 L (1.3 gallons) (liquid) or 5.0 kg (11 lbs) (solid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (1Kg or 1L) |