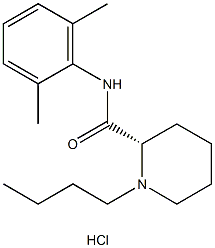
Levobupivacainehydrochloride , 10mMinDMSO , 27262-48-2
Synonym(s):
(S)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide hydrochloride;(S)-bupivacaine hydrochloride;1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide
CAS NO.:27262-48-2
Empirical Formula: C18H29ClN2O
Molecular Weight: 324.89
MDL number: MFCD01704265
EINECS: 1308068-626-2
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB159.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 254 °C (dec.)(lit.) |
| alpha | -12.5 º (c=2, water) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -10 to -14°, c = 1.0 in H2O |
| Water Solubility | H2O: 20mg/mL, clear |
| InChI | InChI=1/C18H28N2O.ClH/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3;/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21);1H/t16-;/s3 |
| InChIKey | SIEYLFHKZGLBNX-NTISSMGPSA-N |
| SMILES | C([C@@H]1CCCCN1CCCC)(=O)NC1C(=CC=CC=1C)C.Cl |&1:1,r| |
| CAS DataBase Reference | 27262-48-2(CAS DataBase Reference) |
Description and Uses
Levobupivacaine was first launched in the US for the production of local anesthesia for surgery and obstetrics and for post-operative pain management. It is the (S)-enantiomer of the long acting, highly potent local anesthetic bupivacaine (Marcaine) that can be prepared by a three step sequence from (S)-pipecolic acid or from (S)-lysine by oxidative deamination and stereospecific ring closure to (S)-pipecolamide core structure. Levobupivacaine exhibits its long-acting local anesthetic effect by blocking neuronal sodium channel ion flow in nerve axons. Clinical studies demonstrated an efficacy and a general profile closely resembling those of the racemic bupivacaine currently in use; however, it produced an enhanced safety profile, in particular substantially reduced (about one-third) cardiotoxicity (less effect on myocardial contractility and QT, prolongation) and CNS depressive side effects. Onset and duration of blockade were also equivalent or even better.
Levobupivacaine hydrochloride has been used as an analyte in tandem mass spectrometry. It may be used to test its inhibitory effect on phosphorylation of extracellular signal-regulated kinase (ERK) mediated by capsaicin It may also be used as a component of poly(D,L-lactide-co-glycolide) (PLGA) microparticles for testing its sustainable release by electrospraying technique.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| Hazard Codes | T+,Xn |
| Risk Statements | 26/27/28-20/21/22-28 |
| Safety Statements | 22-36/37/39-45-36/37-53 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | TK6125000 |