PRODUCT Properties
| Melting point: | 103-105 °C(lit.) |
| Boiling point: | 212 °C10 mm Hg(lit.) |
| Density | 1,329 g/cm3 |
| refractive index | 1.4352 (estimate) |
| Flash point: | 212°C/10mm |
| storage temp. | Store below +30°C. |
| solubility | ethanol: 0.1 g/mL, clear to very faintly hazy |
| pka | 4.71(at 25℃) |
| form | Powder |
| color | White to slightly beige |
| PH | 3.77(1 mM solution);3.25(10 mM solution);2.74(100 mM solution) |
| Water Solubility | 25 g/L (13 ºC) |
| Merck | 14,7431 |
| BRN | 1210024 |
| Stability: | Stable. Incompatible with oxidizing agents, bases. Combustible. |
| InChIKey | WLJVNTCWHIRURA-UHFFFAOYSA-N |
| LogP | 0.610 |
| CAS DataBase Reference | 111-16-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Heptanedioic acid(111-16-0) |
| EPA Substance Registry System | Heptanedioic acid (111-16-0) |
Description and Uses
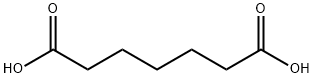
Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H which has been synthesized from cyclohexanone and from salicylic acid. In the former route, the additional carbon is supplied by dimethyloxalate, which reacts with the enolate.
This acid (heptanedioic acid), from the Greek pimelh (pimele fat), as adipic acid, was isolated from oxidized fats. It was obtained in 1884 by Ganttner F et al. as a product of ricinoleic acid (hydroxylated oleic acid) from castor oil.
The major uses of pimelic acid are in plasticizers and polymers.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P304+P340+P312-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 2 |
| RTECS | TK3677000 |
| Hazard Note | Irritant |
| TSCA | Yes |
| HS Code | 29171990 |
| Toxicity | LD50 orally in Rabbit: 7000 mg/kg |