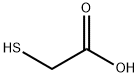
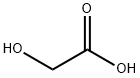
Thioglycolic acid , ≥98%(T) , 68-11-1
Synonym(s):
Mercaptoacetic acid;Mercaptoacetic acid, 2-Mercaptoethanoic acid;Thioglycolic acid
CAS NO.:68-11-1
Empirical Formula: C2H4O2S
Molecular Weight: 92.12
MDL number: MFCD00004876
EINECS: 200-677-4
PRODUCT Properties
| Melting point: | −16 °C(lit.) |
| Boiling point: | 96 °C5 mm Hg(lit.) |
| Density | 1.326 g/mL at 20 °C(lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 0.4 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 126 °C |
| storage temp. | Store at +2°C to +8°C. |
| solubility | Chloroform (Sparingly), Methanol (Sparingly) |
| form | Liquid |
| pka | 3.68(at 25℃) |
| color | clear clear, colorless |
| PH | 1 (H2O, 20℃) |
| Odor | strong unpleasant odor |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,9336 |
| BRN | 506166 |
| Exposure limits | TLV-TWA 1 ppm (~3.8 mg/m3) (ACGIH). |
| Stability: | Air Sensitive |
| InChIKey | CWERGRDVMFNCDR-UHFFFAOYSA-N |
| LogP | 0.090 |
| CAS DataBase Reference | 68-11-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, mercapto-(68-11-1) |
| EPA Substance Registry System | Thioglycolic acid (68-11-1) |
Description and Uses
Thio glycolic acid (TGA) is the organic compound HSCH2CO2H . It contains both a thiol (mercaptan) and a carboxylic acid. It is a clear liquid with a strong unpleasant odor. It is readily oxidized by air to the corresponding disulfide [SCH2CO2H]2.
TGA was developed in the 1940s for use as a chemical depilatory and is still used as such, especially in salt forms, including calcium thioglycolate and sodium thioglycolate. TGA is the precursor to ammonium thioglycolate that is used for permanents. TGA and its derivatives break the disulfide bonds in the cortex of hair. One reforms these broken bonds in giving hair a "perm." Alternatively and more commonly, the process leads to depilation as is done commonly in leather processing. It is also used as an acidity indicator, manufacturing of thioglycolates, and in bacteriology for preparation of thioglycolate media.
TGA is also used in the making of tin stabilizers often used in certain polyvinyl chloride products (such as vinyl siding).
TGA, usually as its dianion, forms complexes with metal ions. Such complexes have been used for the detection of iron, molybdenum, silver, and tin.
Thioglycolic acid is used as nucleophile in thioglycolysis reactions used on condensed tannins to study their structure.
Thioglycolic Acid is an organic compound containing both a thiol and a carboxylic acid. Thioglycolic Acid is a precursor to ammonium thioglycolate, a chemical used for permanents. Thioglycolic Acid is used in organic synthesis as a nucleophile in thioglycolysis reactions and is used as a S transfer agent for sulfonyl chloride synthesis.
Safety
| Symbol(GHS) |   GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H301+H311+H331-H314-H317 |
| Precautionary statements | P261-P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 |
| Hazard Codes | T,T+ |
| Risk Statements | 23/24/25-34-26-24/25 |
| Safety Statements | 25-27-28-45-36/37-28C-23 |
| RIDADR | UN 1940 8/PG 2 |
| OEB | B |
| OEL | TWA: 1 ppm (4 mg/m3) [skin] |
| WGK Germany | 1 |
| RTECS | AI5950000 |
| F | 9-13-23 |
| Autoignition Temperature | 662 °F |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29309070 |
| Hazardous Substances Data | 68-11-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 0.15 ml/kg (Deichmann, Mergard) |