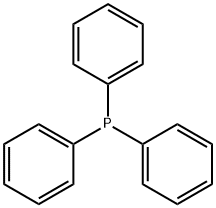
Triphenylphosphine , ≥95% , 603-35-0
Synonym(s):
Phosphorus triphenyl;Phosphorustriphenyl;Triphenylphosphine
CAS NO.:603-35-0
Empirical Formula: C18H15P
Molecular Weight: 262.29
MDL number: MFCD00003043
EINECS: 210-036-0
PRODUCT Properties
| Melting point: | 79-81 °C(lit.) |
| Boiling point: | 377 °C(lit.) |
| bulk density | 500-600kg/m3 |
| Density | 1.132 |
| vapor density | 9 (vs air) |
| vapor pressure | 5 mm Hg ( 20 °C) |
| refractive index | 1.6358 |
| Flash point: | 181 °C |
| storage temp. | Store below +30°C. |
| solubility | water: soluble0.00017 g/L at 22°C |
| form | Crystals, Crystalline Powder or Flakes |
| color | White |
| Specific Gravity | 1.132 |
| Odor | odorless |
| Water Solubility | Insoluble |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9743 |
| BRN | 610776 |
| Stability: | Stable. Incompatible with oxidizing agents, acids. |
| Cosmetics Ingredients Functions | NAIL CONDITIONING |
| InChIKey | RIOQSEWOXXDEQQ-UHFFFAOYSA-N |
| CAS DataBase Reference | 603-35-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphine, triphenyl-(603-35-0) |
| EPA Substance Registry System | Triphenylphosphine (603-35-0) |
Description and Uses
Triphenylphosphine: a member of tertiary phosphines
Triphenylphosphine (TPP) is a member of tertiary phosphines, which is phosphane, in which the three hydrogens are replaced by phenyl groups. It has a role as a reducing agent and an NMR chemical shift reference compound. It is a crucial ligand utilized in the Wittig reaction for alkene synthesis. This reaction involves the formation of alkyliden-etriphenylphosphoranes from the action of butyllithium or another base on the quarternary halide. Triphenylphosphine is used to synthesise organic compounds due to its nucleophilicity and reducing character.
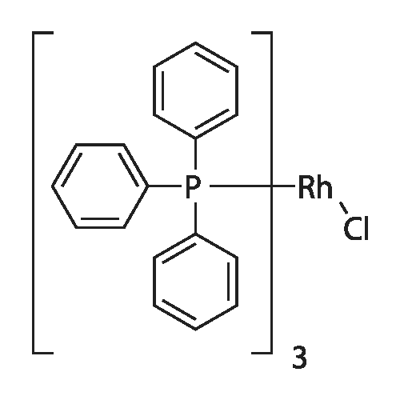
Triphenylphosphine is a versatile and efficient compound with a wide range of applications. It serves as a crucial ligand in homogeneous catalysts for petrochemical and fine chemical production, and as a co-catalyst in the production of isobutanol and n-butanol. It is also the basic raw material for rhodium phosphine complex catalysts, such as Wilkinson's catalyst (RhCl(PPh3)3) for alkene hydrogenation and tetrakis(triphenylphosphine)palladium(0) for C-C coupling reactions in organic synthesis. In the dye industry, it is utilized as a sensitizer, heat stabilizer, light stabilizer, antioxidant, flame retardant, antistatic agent, rubber antiozonant, and analytical reagent. The rhodium and triphenylphosphine catalyst system is employed in the hydroformylation of vegetable oils and their methyl esters, and polymer-supported triphenylphosphine catalyzes the γ-addition of pronucleophiles to alkynoates. It also participates in the Heck reaction of 4-bromoanisole and ethyl acrylate in ionic liquids. Triphenylphosphine can be sulfonated to form trisulfonic acid and is used in Wittig synthesis as a standard ligand in homogeneous catalysis. It is involved in the synthesis of trimethyl phosphite, leading to the production of organophosphorus pesticides like dichlorvos, monocrotophos, and phosphamidon. Furthermore, it is used as a stabilizer in rubber and resin synthesis, an antioxidant in polyvinyl chloride, and a raw material in the synthesis of alkyd resins and polyester resins. Triphenylphosphine is also used in the synthesis of Chlorambucil, a cytotoxic agent for breast and pancreatic cancers, and in the preparation of α-Tocopherol analogues for monitoring antioxidant status.
Safety
| Symbol(GHS) |    GHS05,GHS07,GHS08 |
| Signal word | Danger |
| Hazard statements | H302-H317-H318-H372 |
| Precautionary statements | P260-P280-P301+P312-P302+P352-P305+P351+P338-P314 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-43-53-50/53-48/20/22 |
| Safety Statements | 36/37-60-61-36/37/39-26 |
| RIDADR | 3077 |
| WGK Germany | 2 |
| RTECS | SZ3500000 |
| F | 9 |
| Autoignition Temperature | 425 °C |
| TSCA | Yes |
| HS Code | 29310095 |
| Hazardous Substances Data | 603-35-0(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 700 mg/kg LD50 dermal Rabbit > 4000 mg/kg |