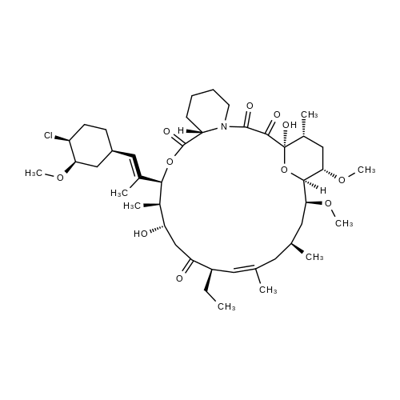
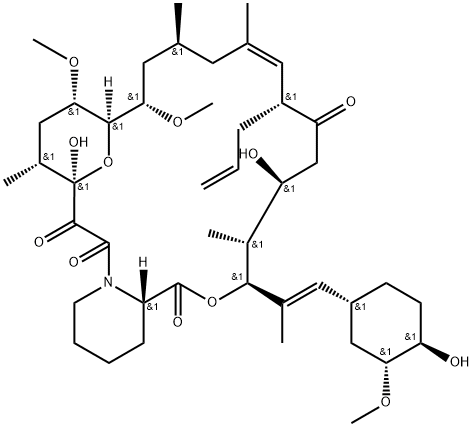
Pimecrolimus , 10mMinDMSO , 137071-32-0
Synonym(s):
33-epi-chloro-33-desoxyascomycin;Picrolimus
CAS NO.:137071-32-0
Empirical Formula: C43H68ClNO11
Molecular Weight: 810.45
MDL number: MFCD00901792
EINECS: 603-999-7
| Pack Size | Price | Stock | Quantity |
| 1ml | RMB559.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 135-136 °C |
| Boiling point: | 866.1±75.0 °C(Predicted) |
| Density | 1.19 |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO (Slightly, Sonicated), Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.97±0.70(Predicted) |
| color | White to Off-White |
Description and Uses
Pimecrolimus is an ascomycin macrolactam derivative, developed as a topical formulation (1% cream) for the treatment of mild to moderate atopic dermatitis for patients aged two years and over in whom the use of conventional therapies is inadvisable. Pimecrolimus is an inflammatory cytokine inhibitor that works by selectively targeting T-cells in the skin. It inhibits in vitro the production and release of pro-inflammatory cytokines after antigen-specific or non-specific stimulation in T cells and mast cells. Pimecrolimus binds specifically to cytosolic receptor macrophilin-12 at nanomolar concentrations leading to inhibition of the Ca2+/calmodulin-dependent phosphatase, calcineurin. Pimecrolimus is a chlorine derivative of the known FK-520, from which it can be synthesized. In a pig model of dinitrofluorobenzene-induced allergic contact dermatitis, pimecrolimus was shown to inhibit erythema and induration, had equivalent efficacy compared to clobetasol-17- propionate, but did not cause atrophogenic effects. In a mouse model of allergic contact dermatitis, pimecrolimus given orally was as potent as tacrolimus and more effective than cyclosporin. The agent also decreased the intensity of cutaneous manifestations in an atopic dermatitis model involving hypomagnesemic hairless mice. Both in adults and in pediatric patients with atopic dermatitis, treatment with pimecrolimus has demonstrated greater efficiency than conventional treatment in reducing the incidence of disease flares, as well as the use of second-line corticosteroids. Moreover, in a 26 week study, in pediatric patients (2-17 years), from 65% of subjects showing improvement, 85% were cleared of the disease. Oral pimecrolimus administration resulted in an elimination half-life of about 30 to 40 h. It is not metabolized or degraded during skin permeation after topical administration. However, following oral administration, it is metabolized via the liver CYP3A4 pathway and excreted mainly in the feces. Pimecrolimus is well tolerated; no systemic accumulation is seen and the most commonly reported side effect is reaction at the site of application. Moreover, pimecrolimus did not cause skin atrophy in contrast to corticosteroids. At this time, as a replacement therapy for topical corticosteroids, the only competing agent for pimecrolimus is topical tacrolimus (ointment). Preliminary studies demonstrated comparable efficacy and safety between these two agents.
Macrolactam ascomycin derivative; inhibits production of pro-inflammatory cytokines by T cells and mast cells. Immunosuppresant Pimecrolimus caused a strong and dose-dependent inhibition of anti-IgE–induced release of histamine from mast cells and basophils (maximally 73% and 82%, respectively, at 500 nmol/L pimecrolimus) and of mast cell tryptase (maximally 75%) and a less pronou.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H332-H312-H302 |
| Precautionary statements | P280-P302+P352-P312-P322-P363-P501-P261-P271-P304+P340-P312-P264-P270-P301+P312-P330-P501 |