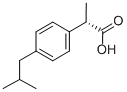
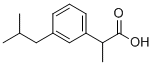
(S)-(+)-Ibuprofen , >98.0%(GC) , 51146-56-6
Synonym(s):
(S)-(+)-2-(4-Isobutylphenyl)propionic acid;(S)-(+)-4-Isobutyl-α-methylphenylacetic acid;S-(+)-IBU
CAS NO.:51146-56-6
Empirical Formula: C13H18O2
Molecular Weight: 206.28
MDL number: MFCD00069289
EINECS: 610-620-9
| Pack Size | Price | Stock | Quantity |
| 1G | RMB28.80 | In Stock |
|
| 5G | RMB36.00 | In Stock |
|
| 25G | RMB115.20 | In Stock |
|
| 100G | RMB434.40 | In Stock |
|
| 500g | RMB2062.40 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 49-53 °C(lit.) |
| alpha | 57 º (c=2, EtOH) |
| Boiling point: | 285.14°C (rough estimate) |
| Density | 1.0364 (rough estimate) |
| refractive index | 59 ° (C=2, EtOH) |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.5 mg/mL |
| pka | 4.41±0.10(Predicted) |
| form | solid |
| color | white |
| optical activity | [α]20/D +59°, c = 2 in ethanol |
| Water Solubility | insoluble |
| BRN | 3590022 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | HEFNNWSXXWATRW-JTQLQIEISA-N |
| CAS DataBase Reference | 51146-56-6(CAS DataBase Reference) |
Description and Uses
Dexibuprofen, the S-(+)-isomer of the widely used NSAID agent ibuprofen, was launched in Austria for the treatment of rheumatoid arthritis. While the racemic compound is commonly used clinically, the antiinflammatory activity is mediated via the S-isomer by inhibition of prostaglandin synthesis. It has also been demonstrated that the R-isomer is converted to the Santipode in vivo via a CoA thioester intermediate. Since CoA plays a pivotal role in intermediary metabolism and maintenance of the [acyl-CoA], generation of R-ibuprofen-CoA competitively inhibits many CoA-dependent reactions, which consequently produces perturbations of hepatocyte Intermediary metabolism and mitocondrial function. Pure S-ibuprofen usage, therefore, is preferred allowing a reduction in dosage level and an improved side effect profile.
Ibuprofen is a non-steroidal anti-inflammatory drug with diverse biochemical actions, most notably inhibiting COX-1 and COX-2 (IC50s = 2.6 and 1.53, μM, respectively). It is commonly synthesized as a racemic mixture of (S)- and (R)-isomers. (S)-Ibuprofen is an enantiomer that more potently inhibits COX activity, thromboxane formation, and platelet aggregation than the (R)-form. (S)-Ibuprofen also inhibits activation of NF-κB more effectively than (R)-ibuprofen (IC50s = 62 and 122 μM, respectively). However, the enantiomers are equipotent in blocking superoxide formation, β-glucuronidase release, and LTB4 generation by stimulated neutrophils (IC50 values range from 0.14 to 0.58 μM). A majority of (R)-ibuprofen can be inverted to (S)-ibuprofen in humans after oral administration.