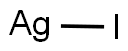PRODUCT Properties
| Melting point: | 557°C |
| Boiling point: | 1506°C |
| Density | 5.68 g/mL at 25 °C (lit.) |
| RTECS | VW4450000 |
| solubility | insoluble in acid solutions |
| form | Solid |
| Specific Gravity | 6.01 |
| color | Yellow |
| Odor | odorless |
| Water Solubility | 0.03 mg/L |
| Sensitive | Light Sensitive |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,8516 |
| Solubility Product Constant (Ksp) | pKsp: 16.07 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Stability: | Stability Light-sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7783-96-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Silver iodide(7783-96-2) |
| EPA Substance Registry System | Silver iodide (AgI) (7783-96-2) |
Description and Uses
Silver iodide is a yellow powder formed by the combination of a soluble iodide combined with silver nitrate. Silver iodide could also be formed by exposing metallic silver to the fumes of bromine as in the daguerreotype process. This was the primary halide used for all of the 19th century camera processes until the introduction of the silver bromide gelatin plate. With the exception of the daguerreotype, all silver iodide processes relied on physical development using a reducing agent such as gallic acid, pyrogallic acid, or ferrous sulfate, an acid restrainer, and excess silver.
Safety
| Symbol(GHS) |  GHS09 |
| Signal word | Warning |
| Hazard statements | H410-H400 |
| Precautionary statements | P273-P501 |
| Risk Statements | 36/37/38-53 |
| Safety Statements | 22-24/25 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| F | 8 |
| TSCA | Yes |
| HazardClass | 9 |
| HS Code | 28432900 |
| Hazardous Substances Data | 7783-96-2(Hazardous Substances Data) |