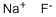Sodium fluoride , 99.99%metalsbasis , 7681-49-4
Synonym(s):
Sodium fluoride;Sodium fluoride solution;Sodium monofluoride;Sodium monofluoride (NaF);Disodium Fluoride
CAS NO.:7681-49-4
Empirical Formula: FNa
Molecular Weight: 41.99
MDL number: MFCD00003524
EINECS: 231-667-8
PRODUCT Properties
| Melting point: | 993 °C (lit.) |
||||||||||||||
| Boiling point: | 1700 °C |
||||||||||||||
| Density | 1.02 g/mL at 20 °C |
||||||||||||||
| vapor pressure | 1.4 mm Hg ( 0 °C) |
||||||||||||||
| refractive index | 1.336 |
||||||||||||||
| Flash point: | 1704°C |
||||||||||||||
| storage temp. | 2-8°C |
||||||||||||||
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
||||||||||||||
| form | powder |
||||||||||||||
| color | White to off-white |
||||||||||||||
| Specific Gravity | 2.558 |
||||||||||||||
| Odor | Odorless |
||||||||||||||
| PH | 7.0-10.0 (25℃, 0.5M in H2O) |
||||||||||||||
| Water Solubility | 4 g/100 mL (25 ºC) |
||||||||||||||
| Sensitive | Hygroscopic |
||||||||||||||
| Crystal Structure | NaCl type |
||||||||||||||
| crystal system | Cube |
||||||||||||||
| Merck | 14,8618 |
||||||||||||||
| Space group | Fm3m |
||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
||||||||||||||
| Stability: | Stable. Hydrolyzed by water. Reacts with mineral acids to generate highly toxic hydrogen fluoride. Incompatible with glass. |
||||||||||||||
| CAS DataBase Reference | 7681-49-4(CAS DataBase Reference) |
||||||||||||||
| NIST Chemistry Reference | Sodium fluoride(7681-49-4) |
||||||||||||||
| EPA Substance Registry System | Sodium fluoride (7681-49-4) |
Description and Uses
Sodium fluoride, NaF, is a binary salt that is a clear, lustrous crystal or white powder. The insecticide grade is frequently dyed blue. It is soluble in water and has a specific gravity of 2.558, which is heavier than water. Sodium fluoride is highly toxic by ingestion and inhalation, and is also strongly irritating to tissue. The TLV is 2.5 mg/m3 of air. The four-digit UN identification number is 1690. The primary uses are fluoridation of municipal water at 1 ppm, as an insecticide, rodenticide, and fungicide, and in toothpastes and disinfectants.
Sodium fluoride is a versatile chemical reagent widely used in the fields of chemical industry, medicine, food, materials and electrochemistry. It can be used as a reagent in analytical chemistry and biological experiments (such as protein and enzyme analysis); adding 1 ppm NaF to drinking water can be used to prevent dental caries. It is also used to make adhesives, disinfectants and dental products, or for pesticide formulation components; used as a food preservative, such as preservatives and anti-fermentation agents in alcohol distilleries; used as a component of ceramic enamel and flux, or to make coated paper and frosted glass; used to preserve animal specimens.
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301-H315-H319 |
| Precautionary statements | P264-P270-P280-P301+P310-P302+P352-P305+P351+P338 |
| Hazard Codes | T,Xn |
| Risk Statements | 25-32-36/38-22 |
| Safety Statements | 23-24/25-45-36-22 |
| RIDADR | UN 1690 6.1/PG 3 |
| OEB | B |
| OEL | TWA: 2.5 mg/m3 [*Note: The REL also applies to other inorganic, solid fluorides (as F).] |
| WGK Germany | 1 |
| RTECS | WB0350000 |
| F | 10 |
| Hazard Note | Toxic/Hygroscopic |
| TSCA | Yes |
| HS Code | 2826 19 10 |
| HazardClass | 6.1 |
| PackingGroup | III |
| Hazardous Substances Data | 7681-49-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 0.18 g/kg (Smyth) |